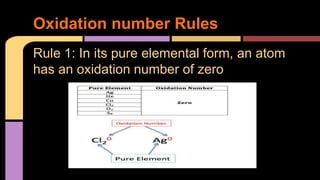
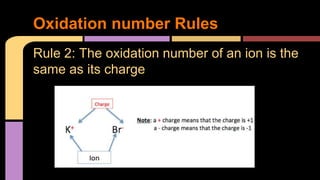
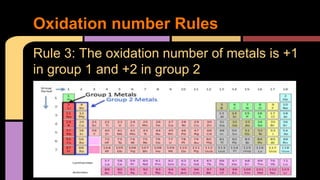
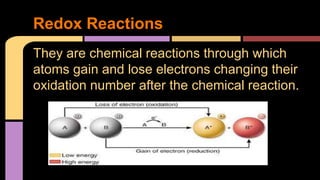
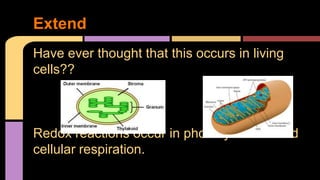
The document explains oxidation as a chemical reaction process involving the loss of electrons, particularly in reactions with oxygen. It outlines oxidation number rules and provides examples of how to determine oxidation states in compounds and ions. Additionally, it discusses redox reactions, which involve the gain and loss of electrons, and their significance in biological processes like photosynthesis and cellular respiration.


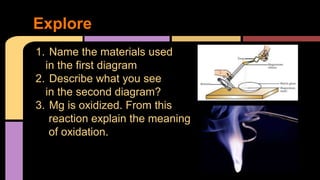

![Oxidation Number
It indicates if electrons are lost or gained by
an atom during a chemical reaction.
2Mg0 + O0
2 2 [ Mg+2 O-2]
Mg lost 2 electrons while Oxygen gained 2
electrons. The number above each atom/ion
is its oxidation number.](https://image.slidesharecdn.com/oxidationreductionlessongrade9-141206182913-conversion-gate02/85/Oxidation-reduction-lesson-grade-9-5-320.jpg)