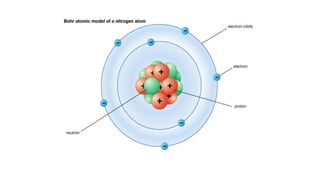
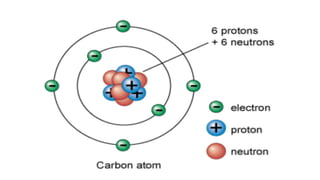
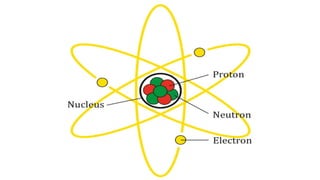
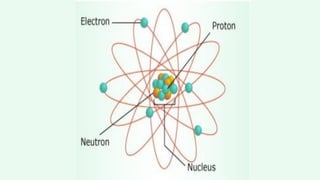
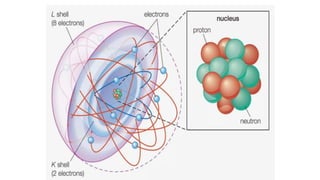
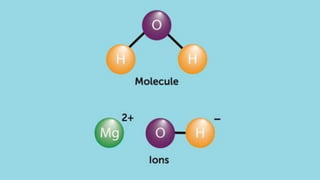
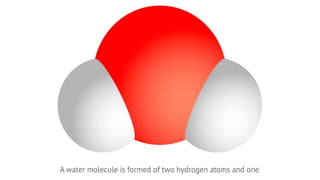
- An atom is the smallest unit of an element, and two or more atoms bonded together form a molecule. Molecules can be made of two or more identical atoms (such as oxygen, O2), or different elements (such as water, H2O).
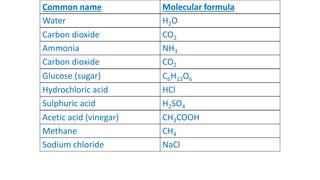
- A compound is a molecule consisting of different types of atoms, while an element is made of only one type of atom. Common compounds include water, carbon dioxide, and table sugar.
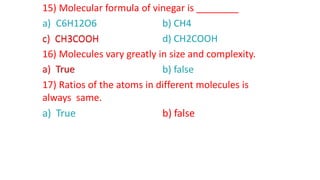
- Molecular formulas use symbols to represent the elements and subscripts to show the number of atoms in a molecule, such as H2O for a water molecule. The number and type of atoms can vary between molecules of the same substance.