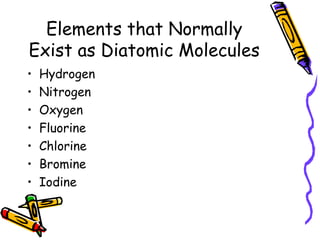
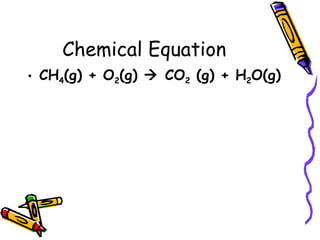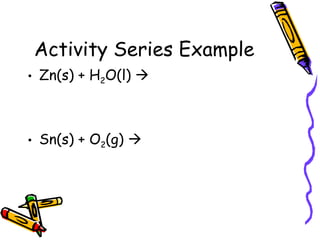The document discusses chemical equations and reactions, including:
- Indications that a chemical reaction has occurred include evolution of energy (heat/light), production of a gas, and color change.
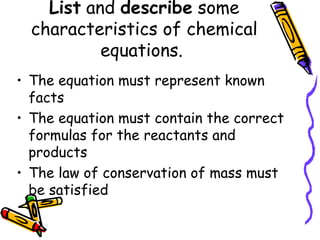
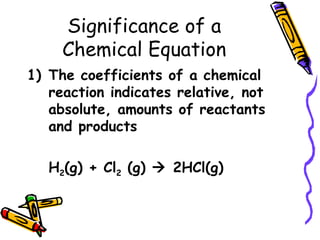
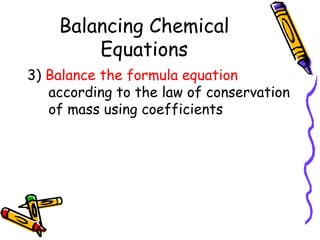
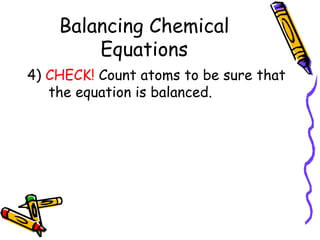
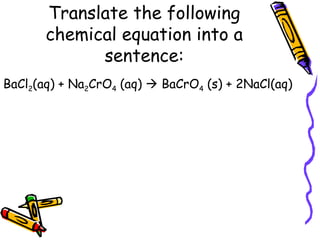
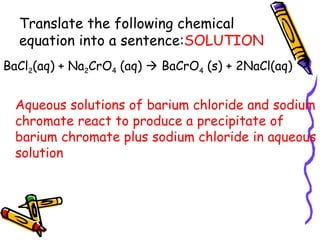
- Chemical equations must represent known facts, contain correct formulas, and satisfy the law of conservation of mass.
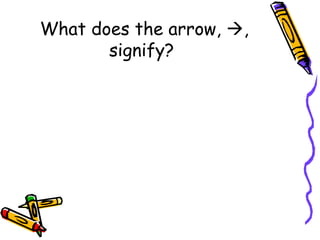
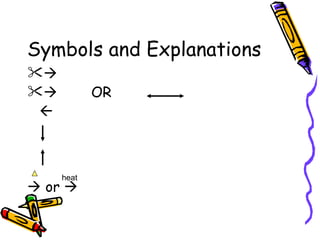
- The arrow in an equation signifies a reaction occurring. Equations can show reversible reactions with double arrows.
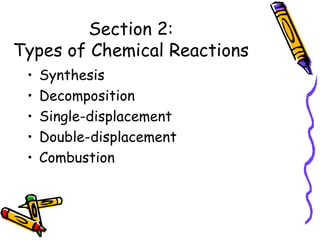
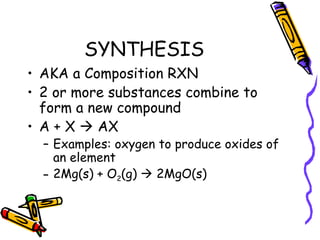
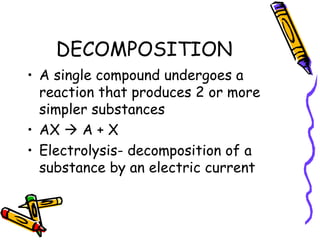
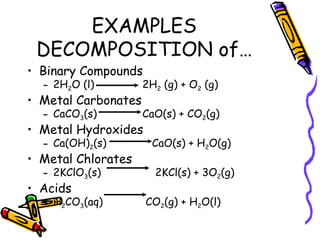
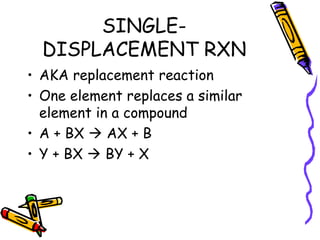
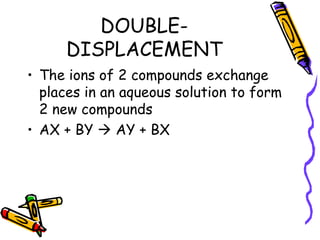
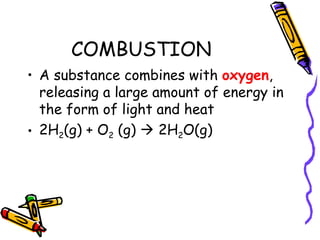
- Types of chemical reactions include synthesis, decomposition, single-displacement, double-displacement, and combustion.
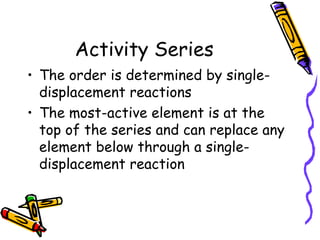
- The activity series lists elements in order of their reactivity based on displacement reactions, and can predict if a reaction will occur.