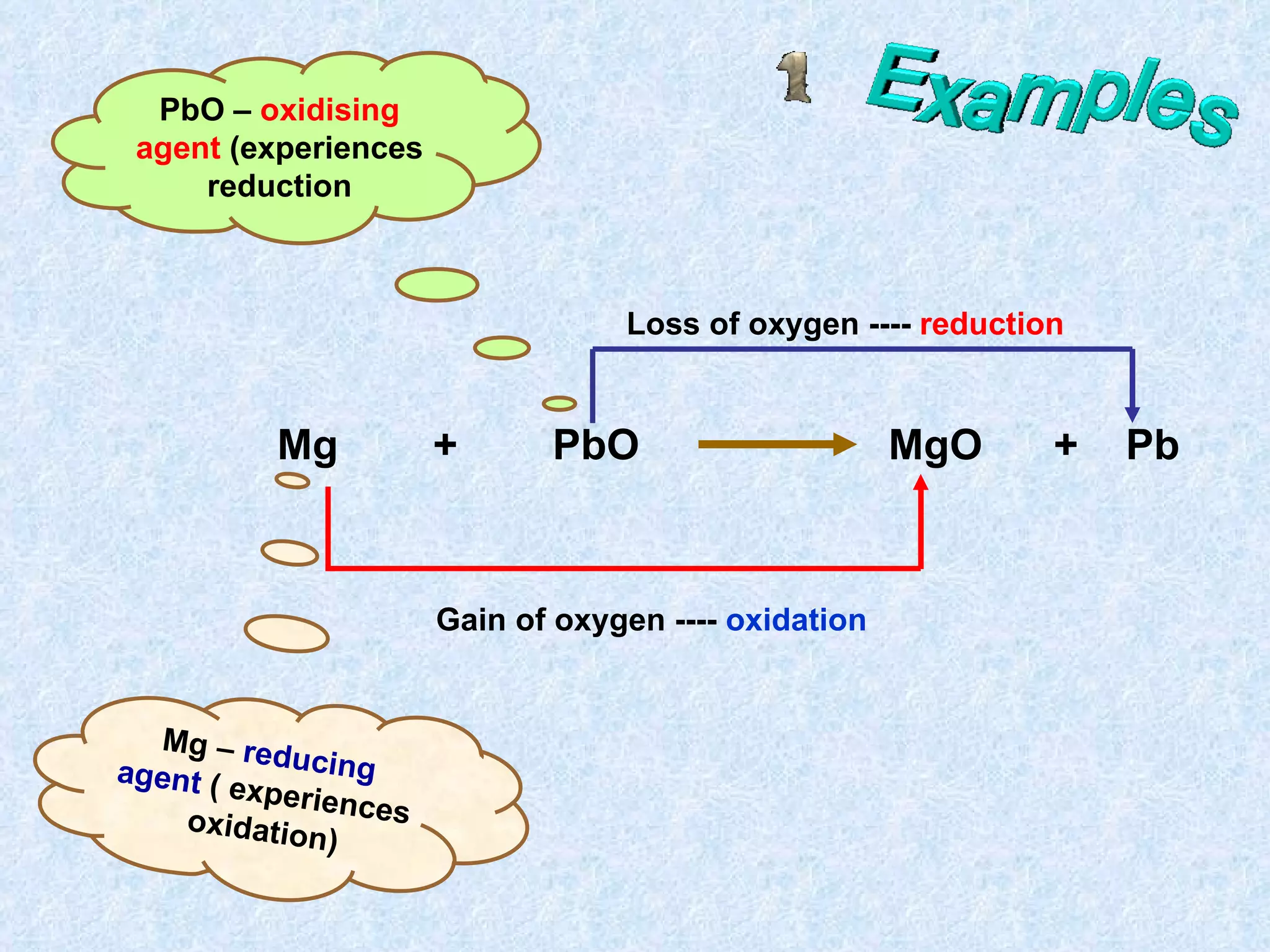
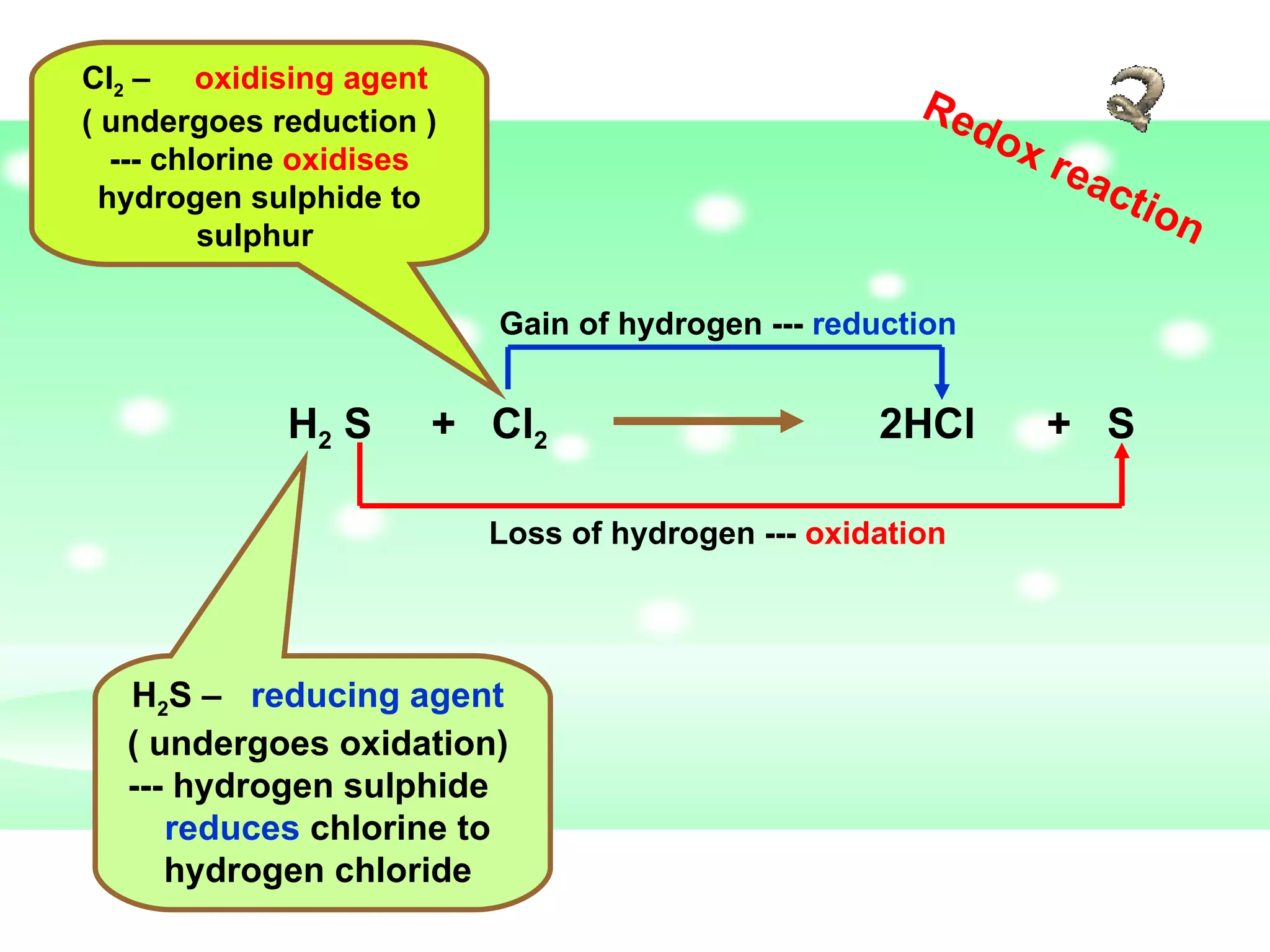
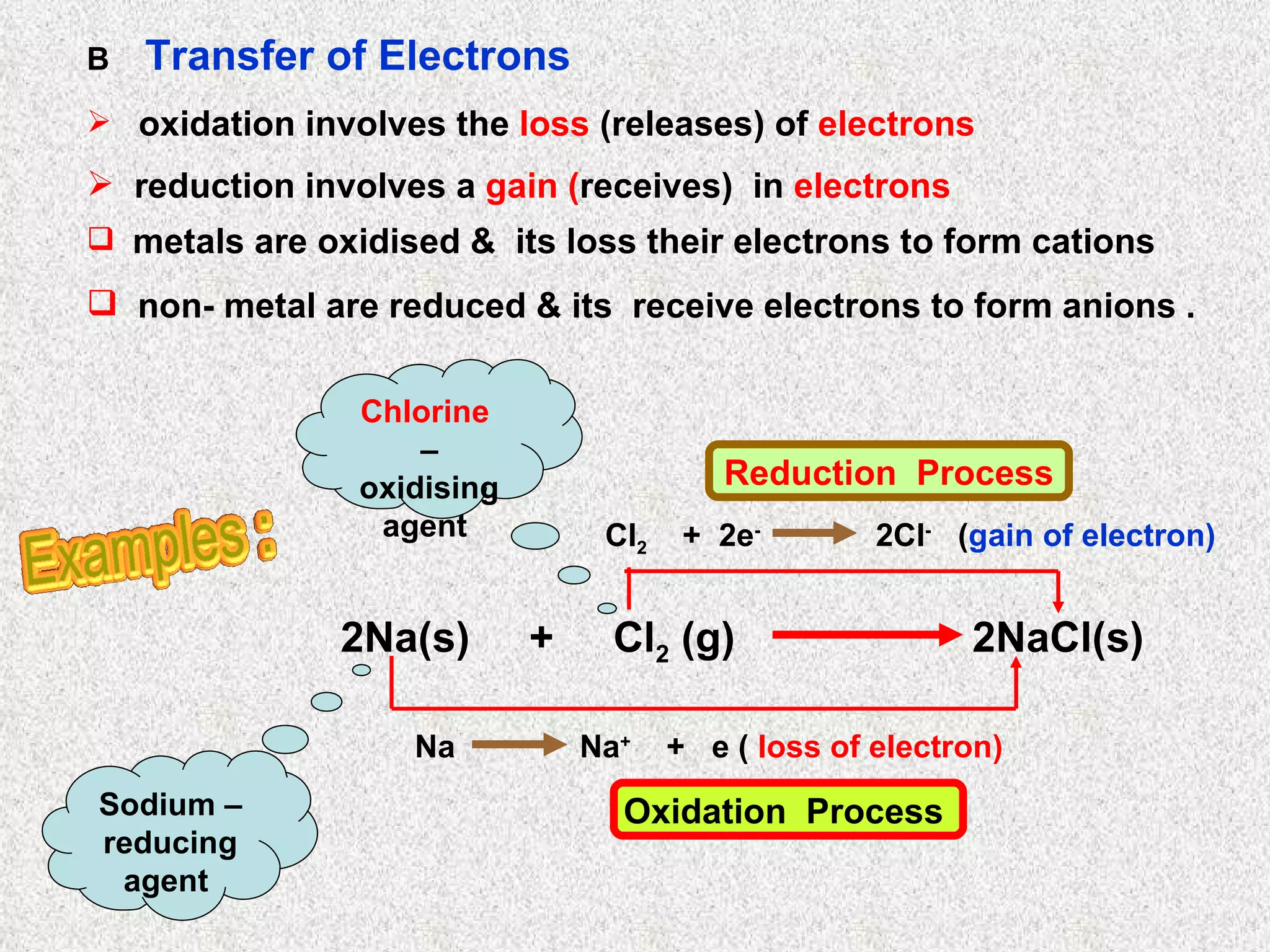
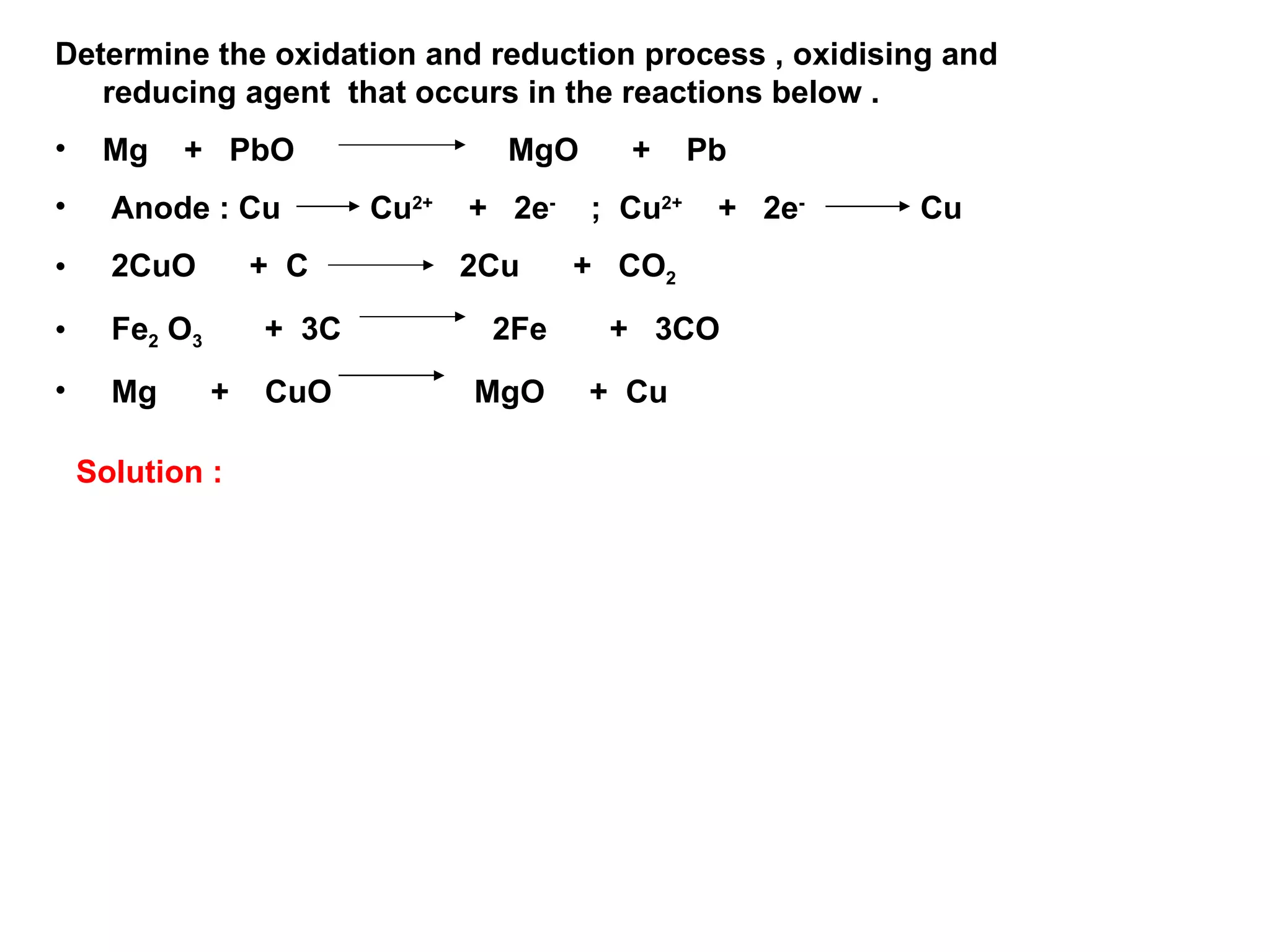
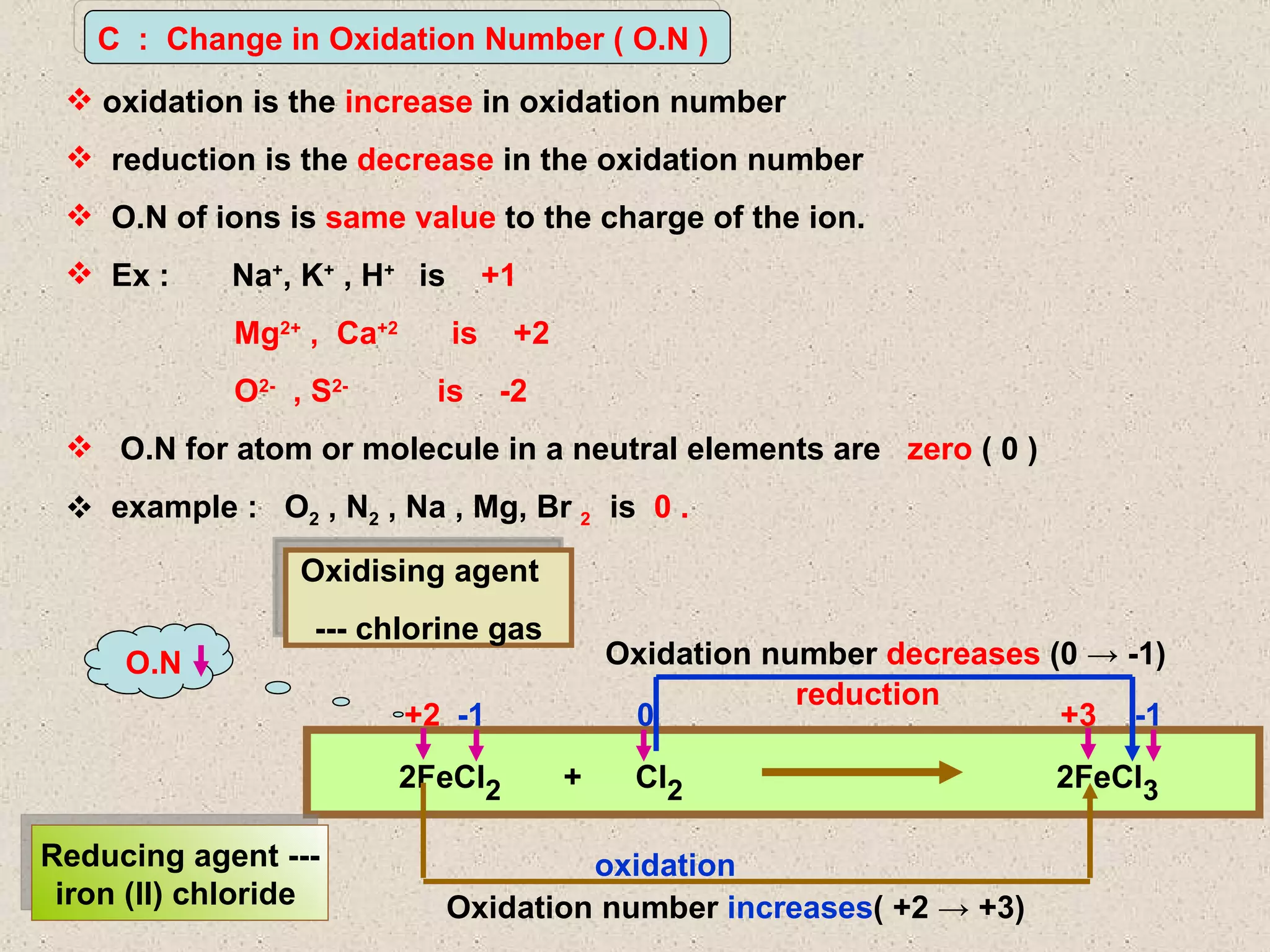
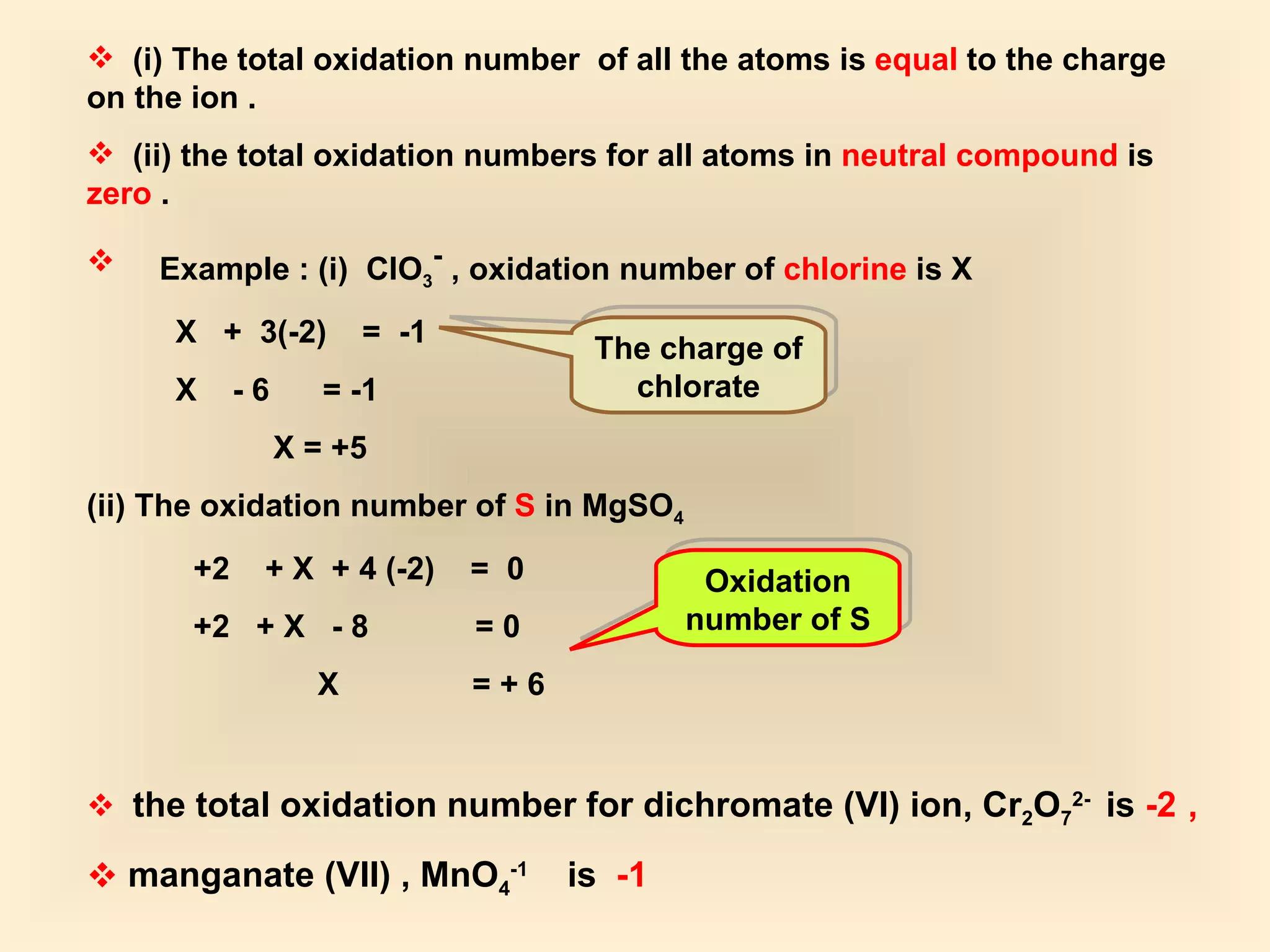
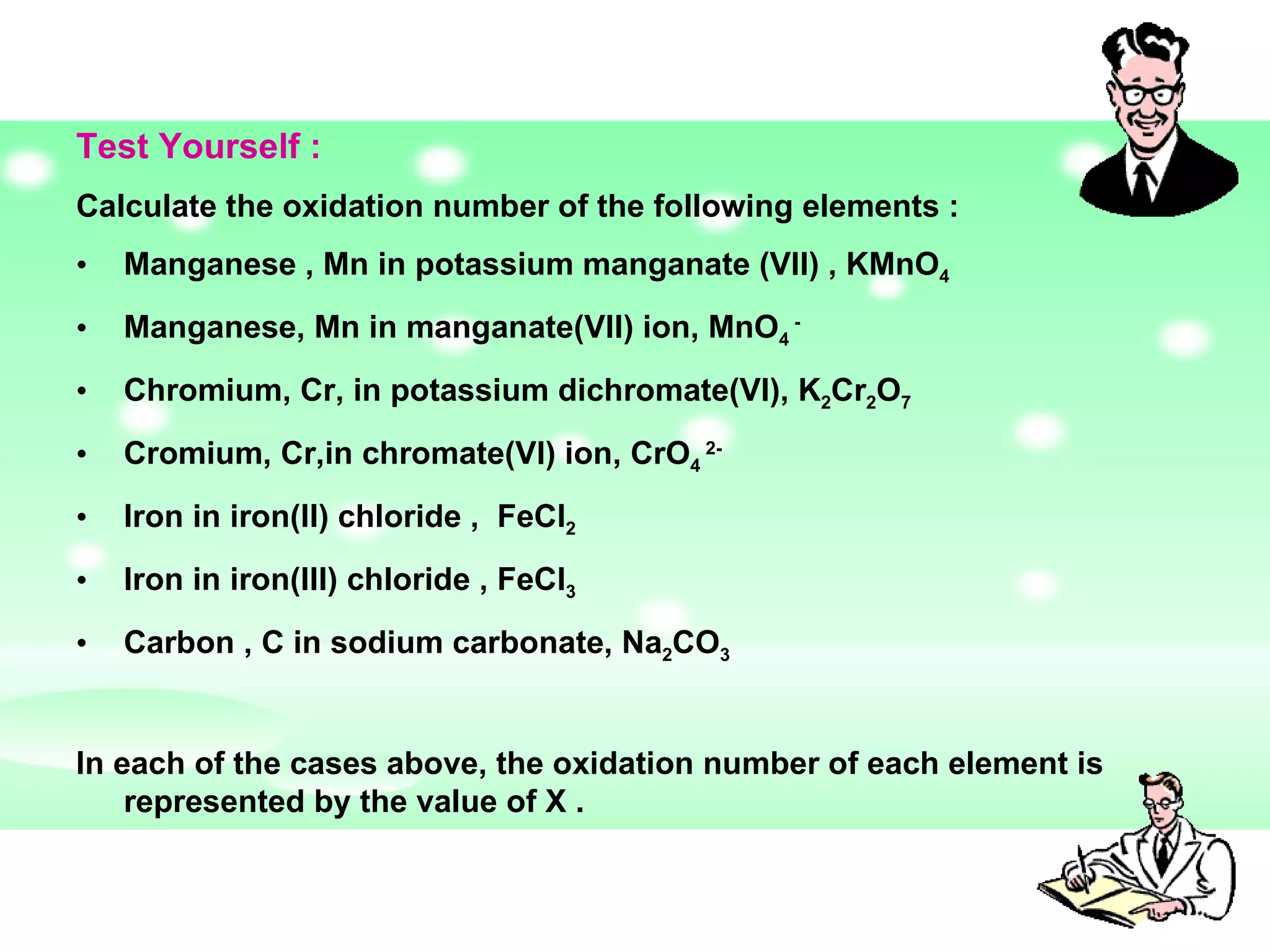
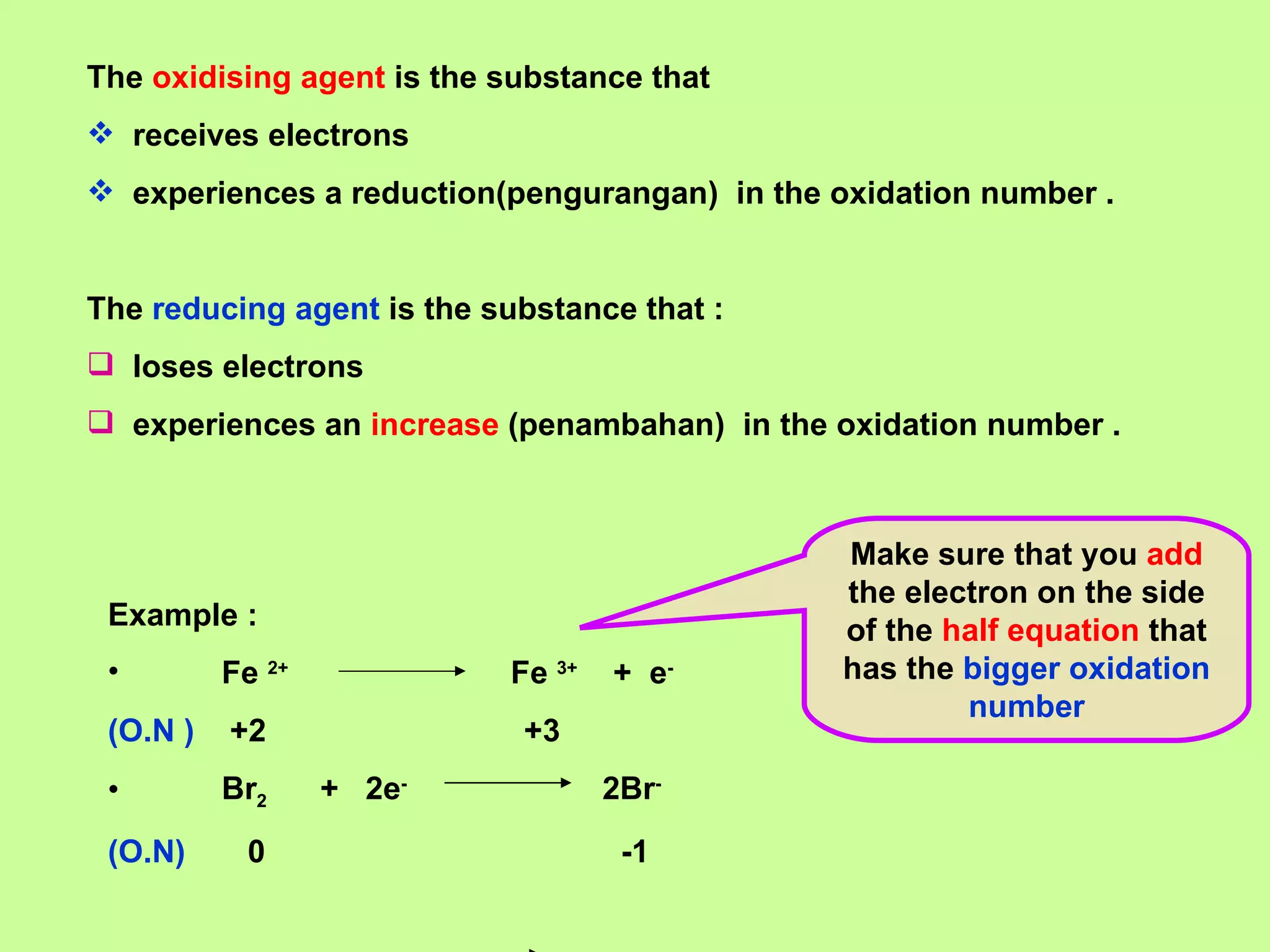
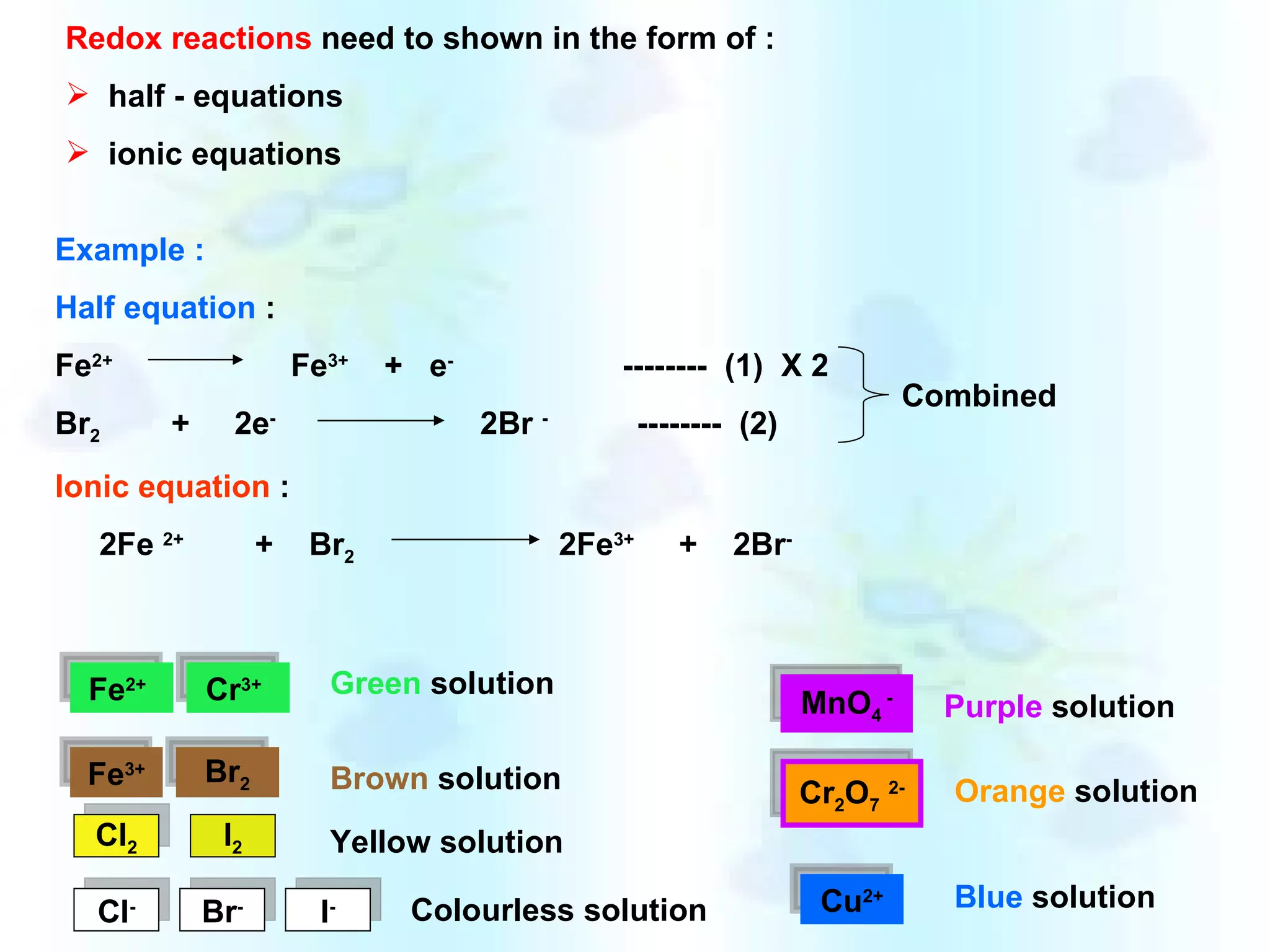
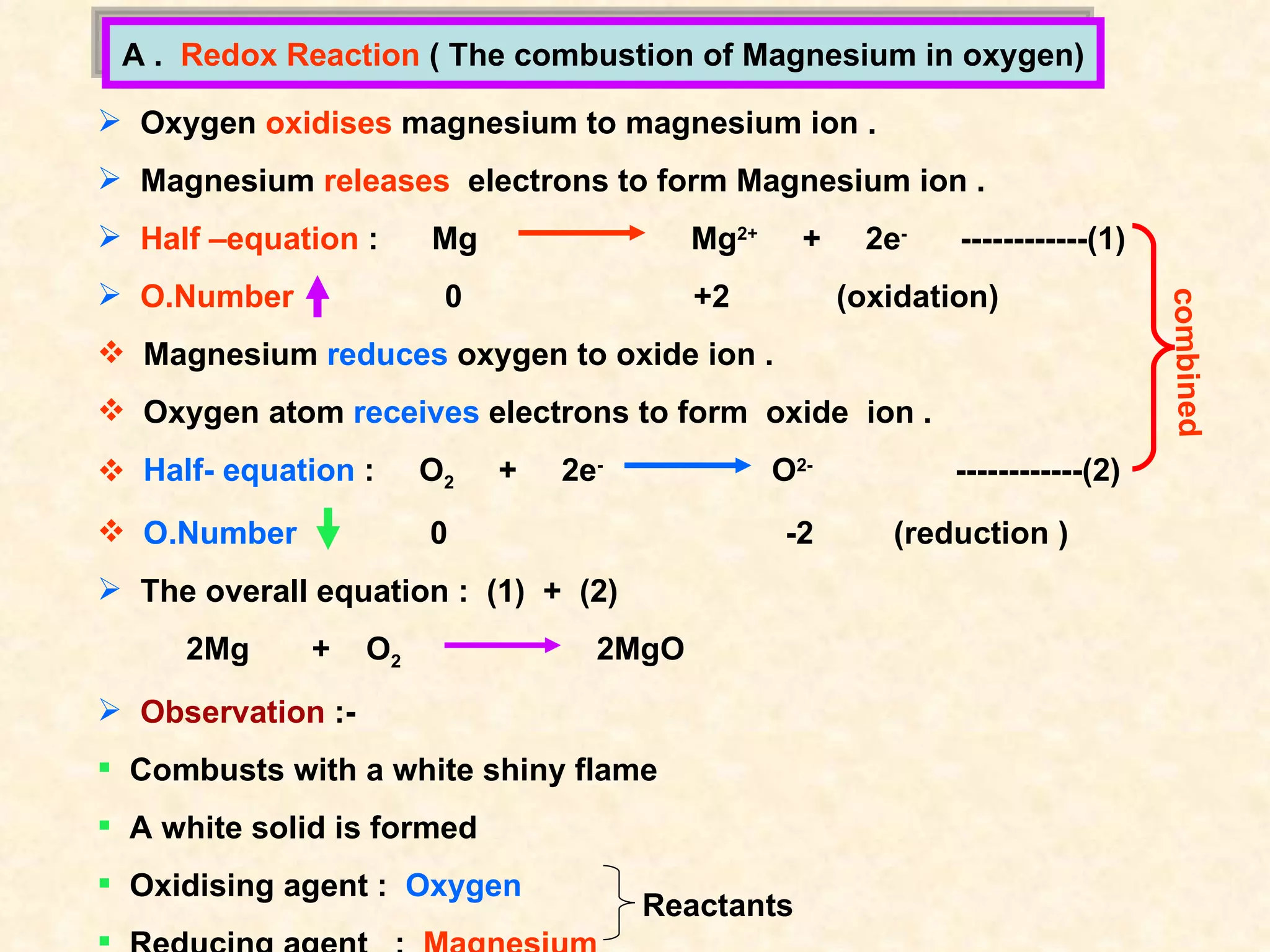
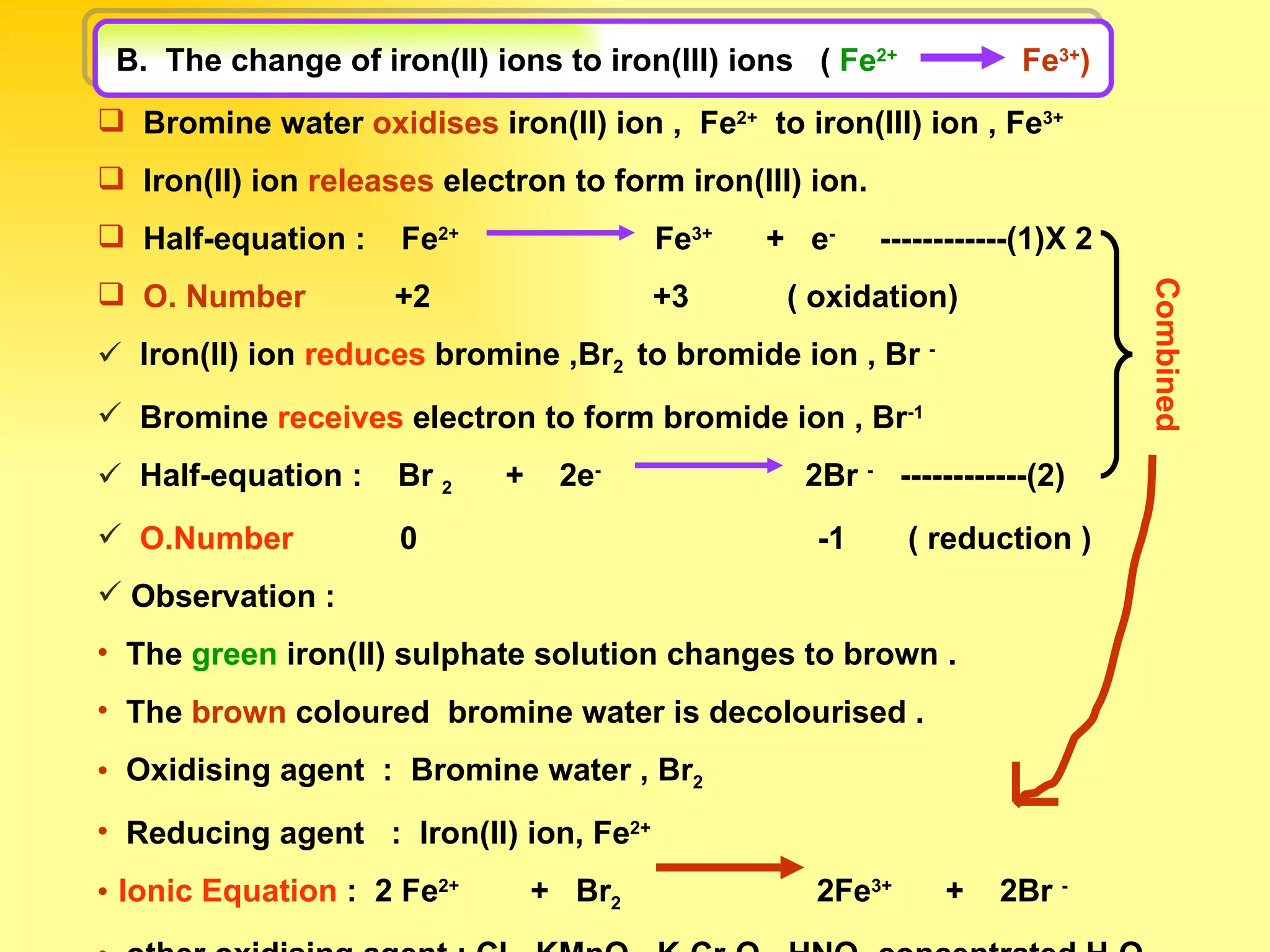
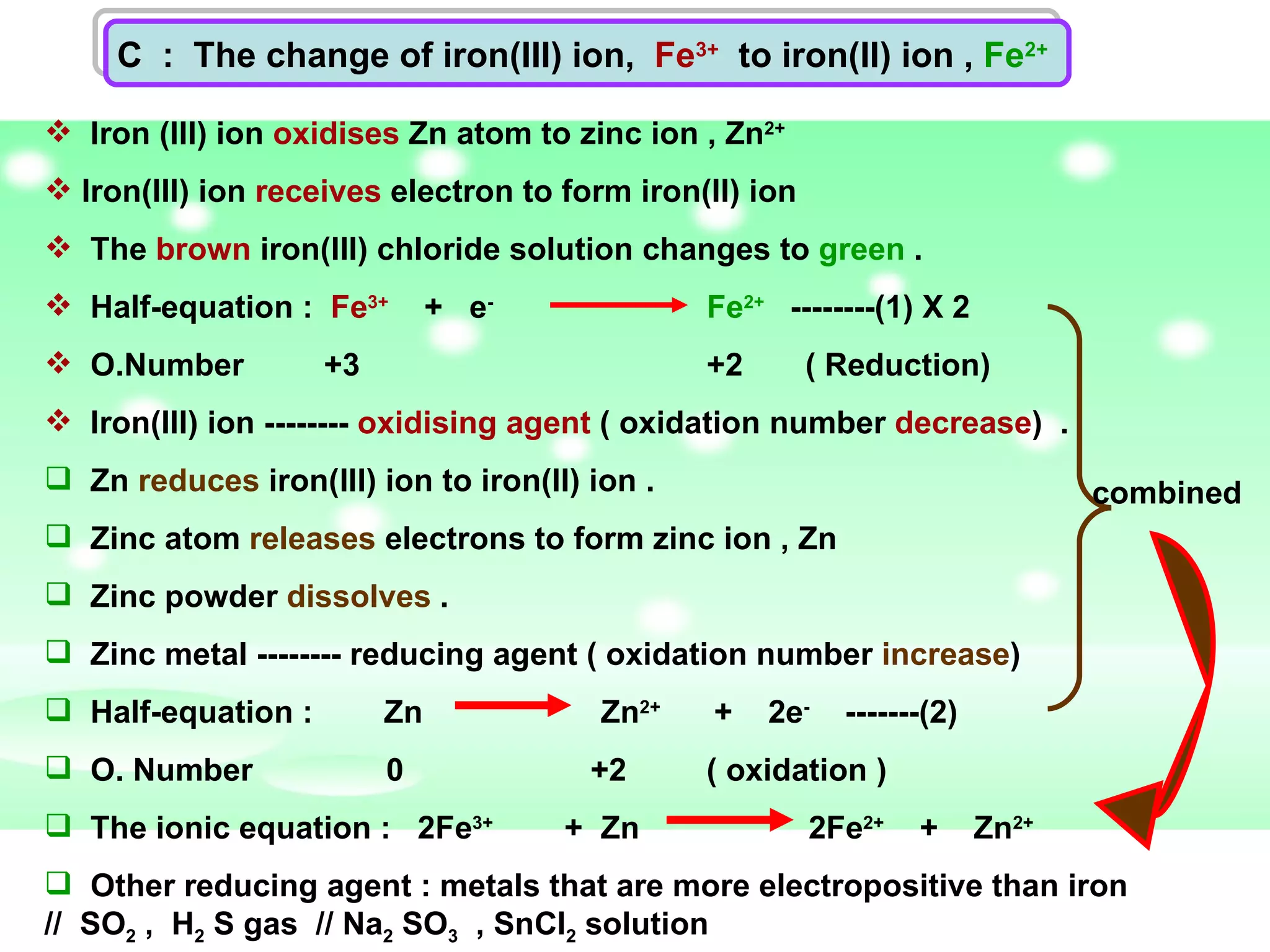
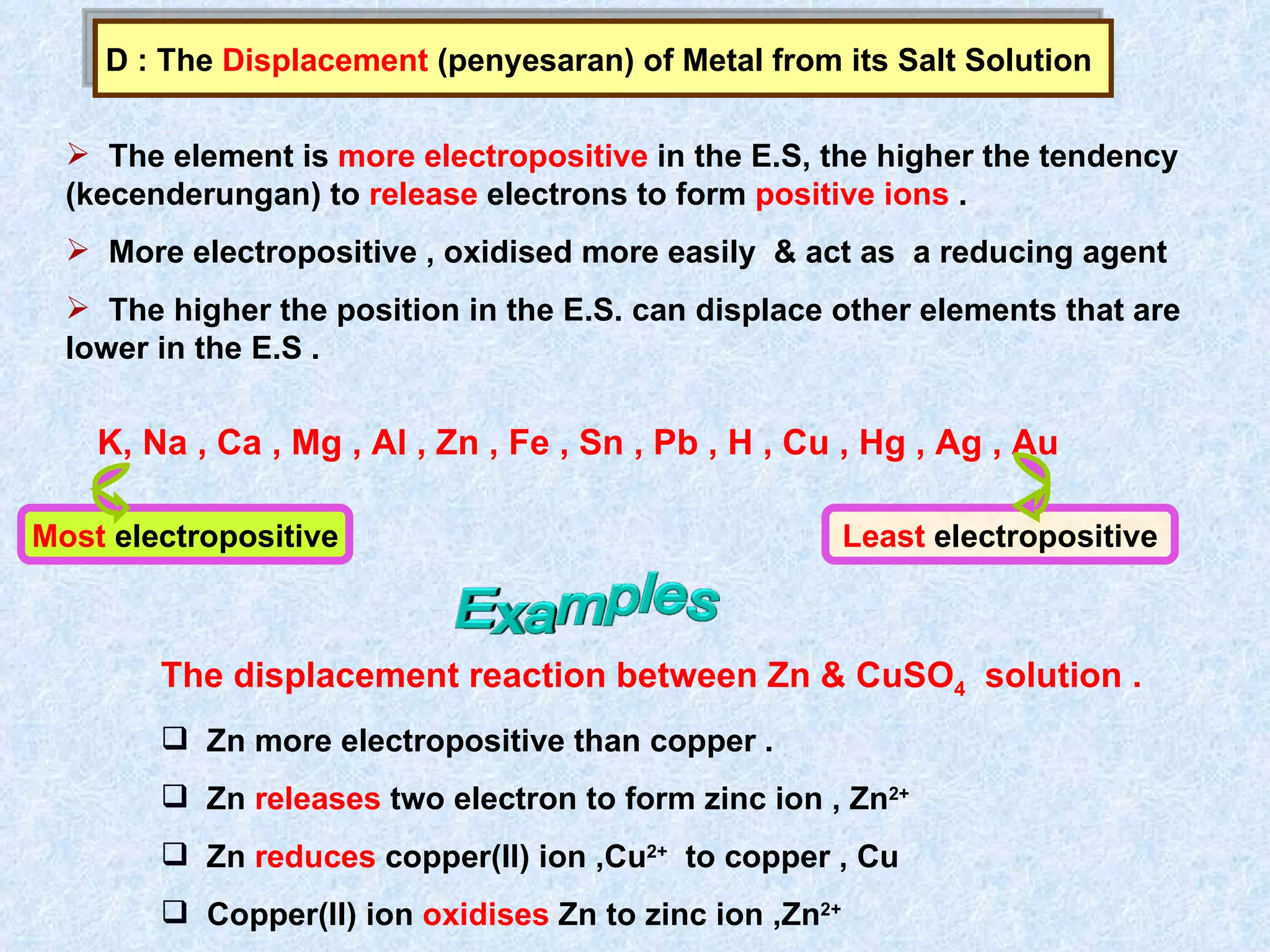
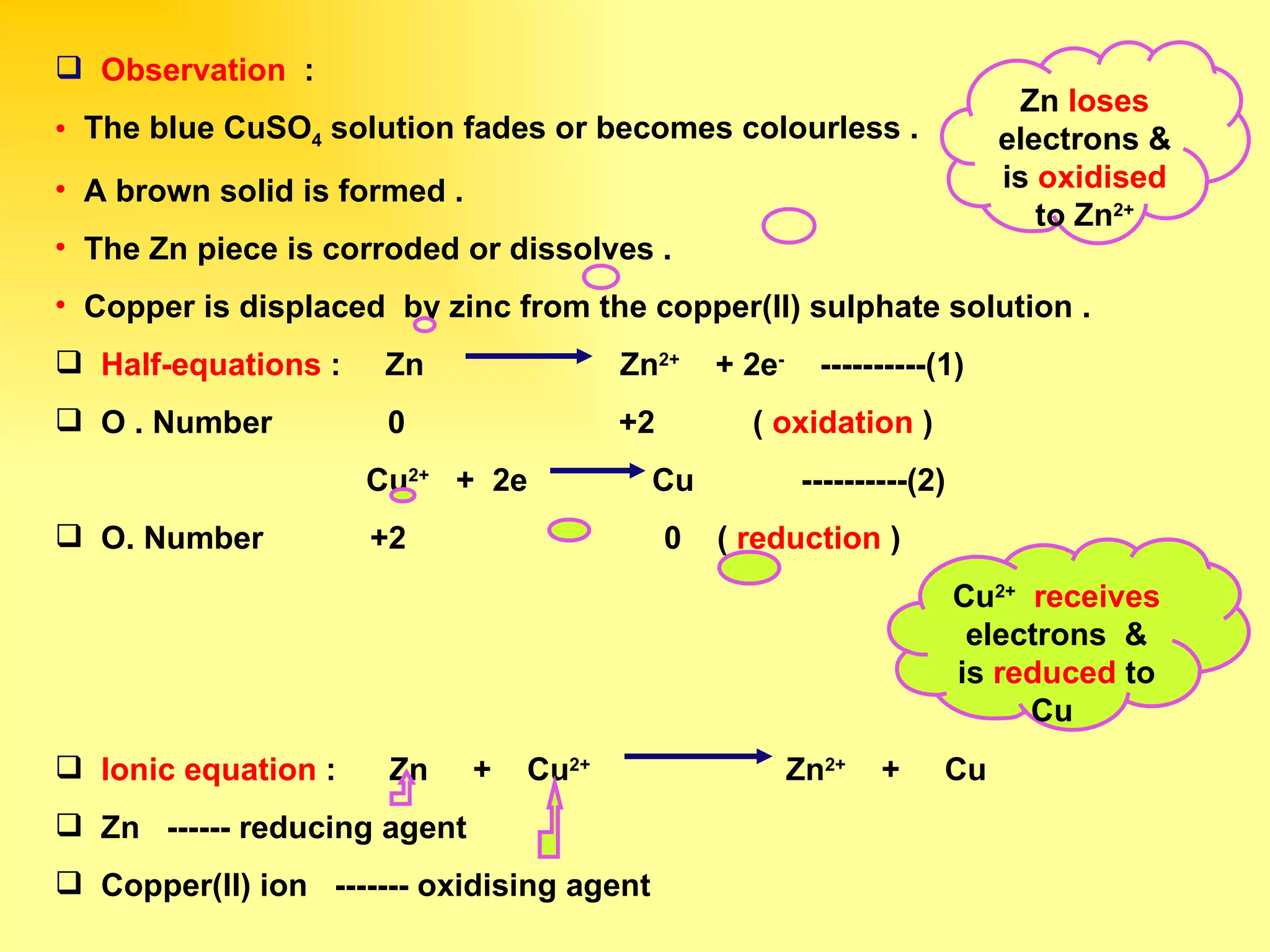
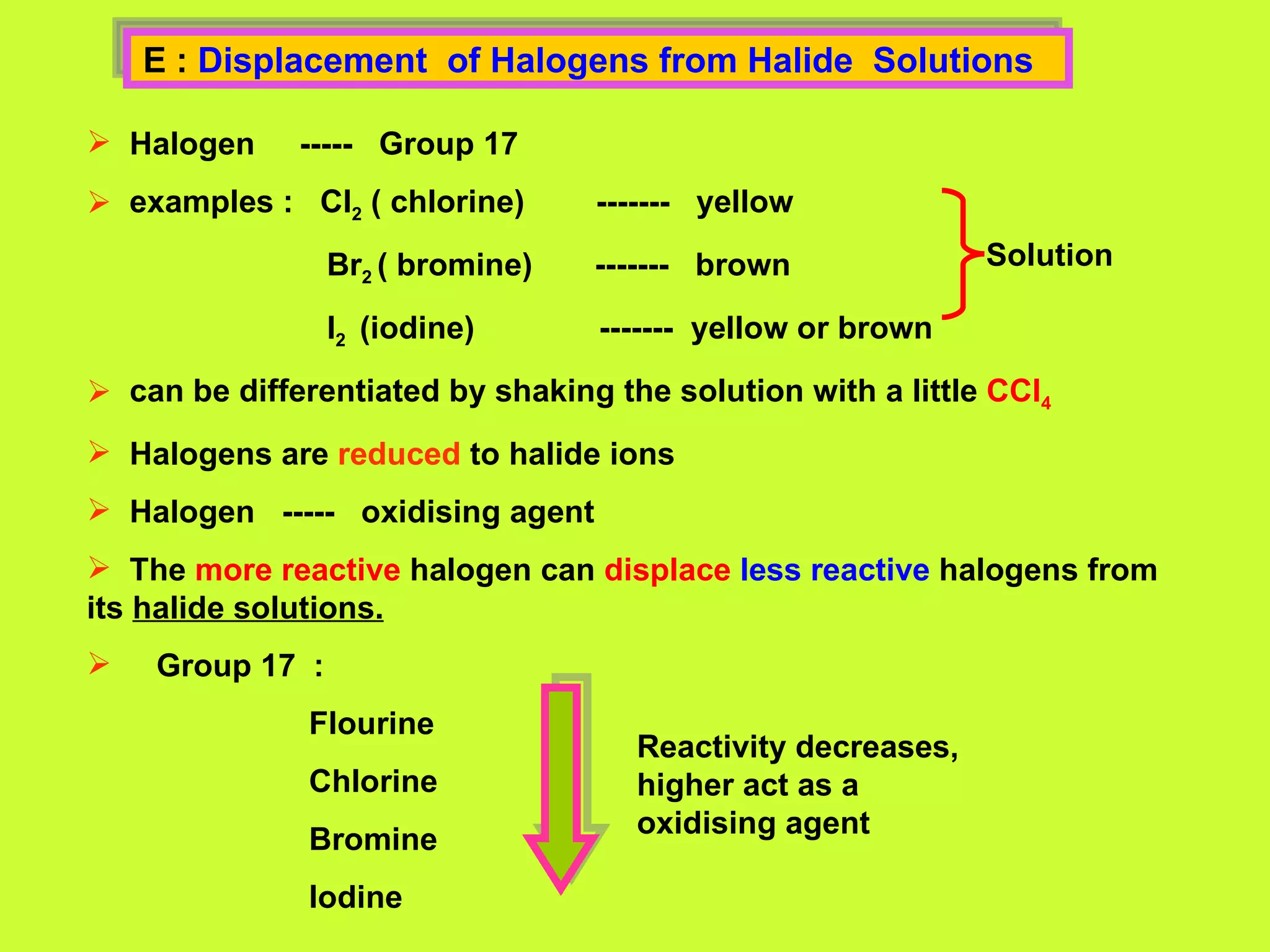
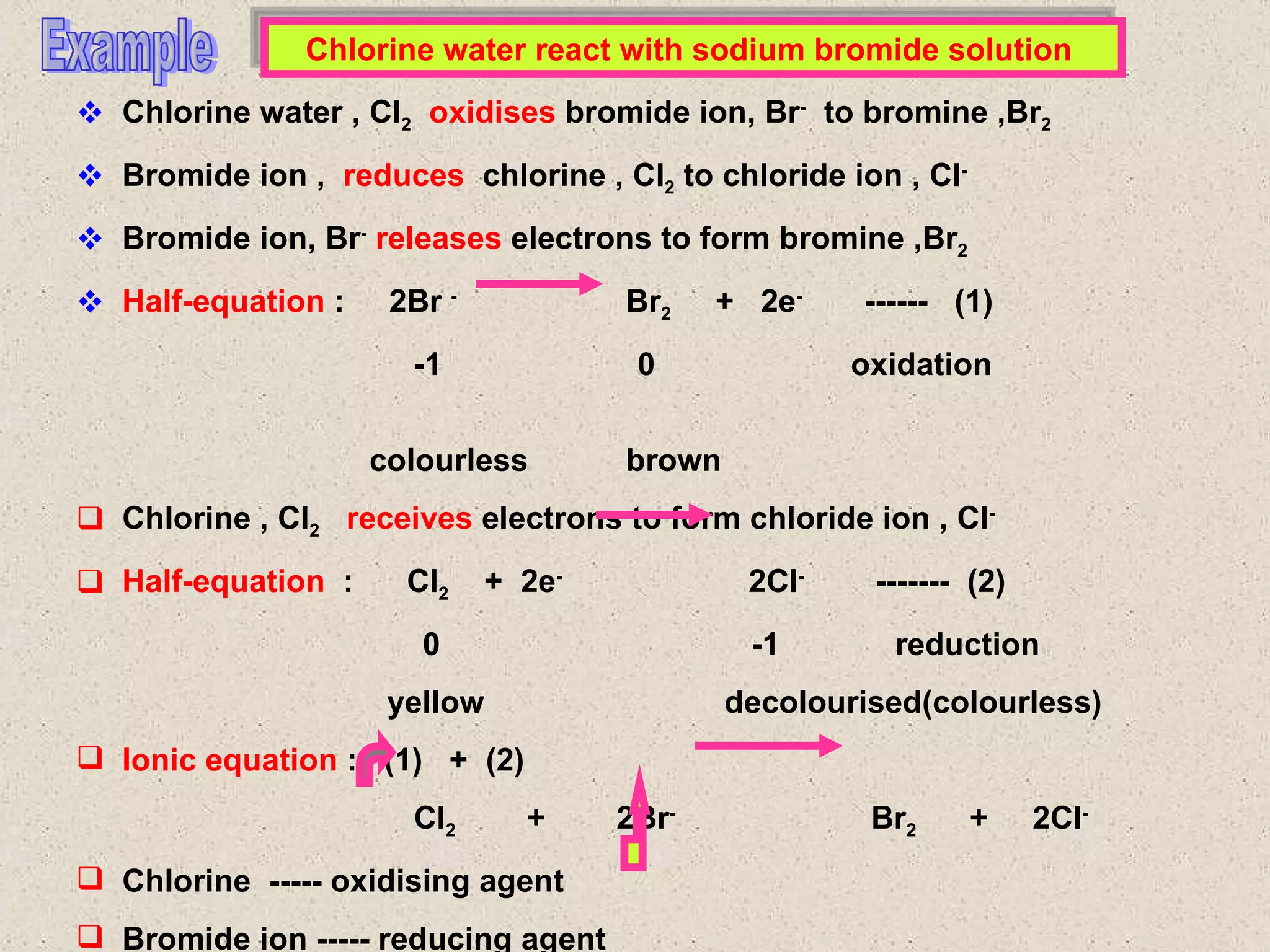
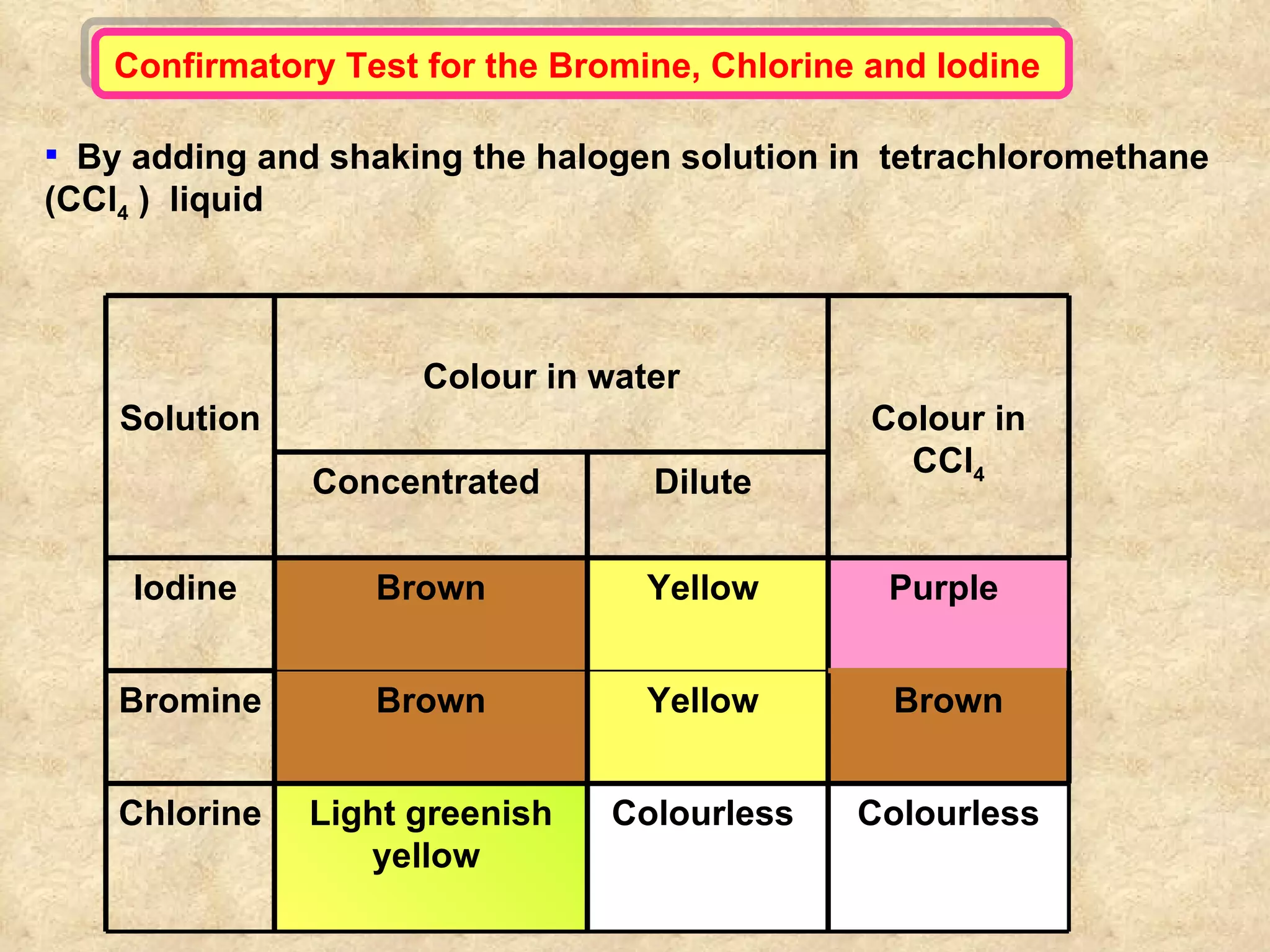
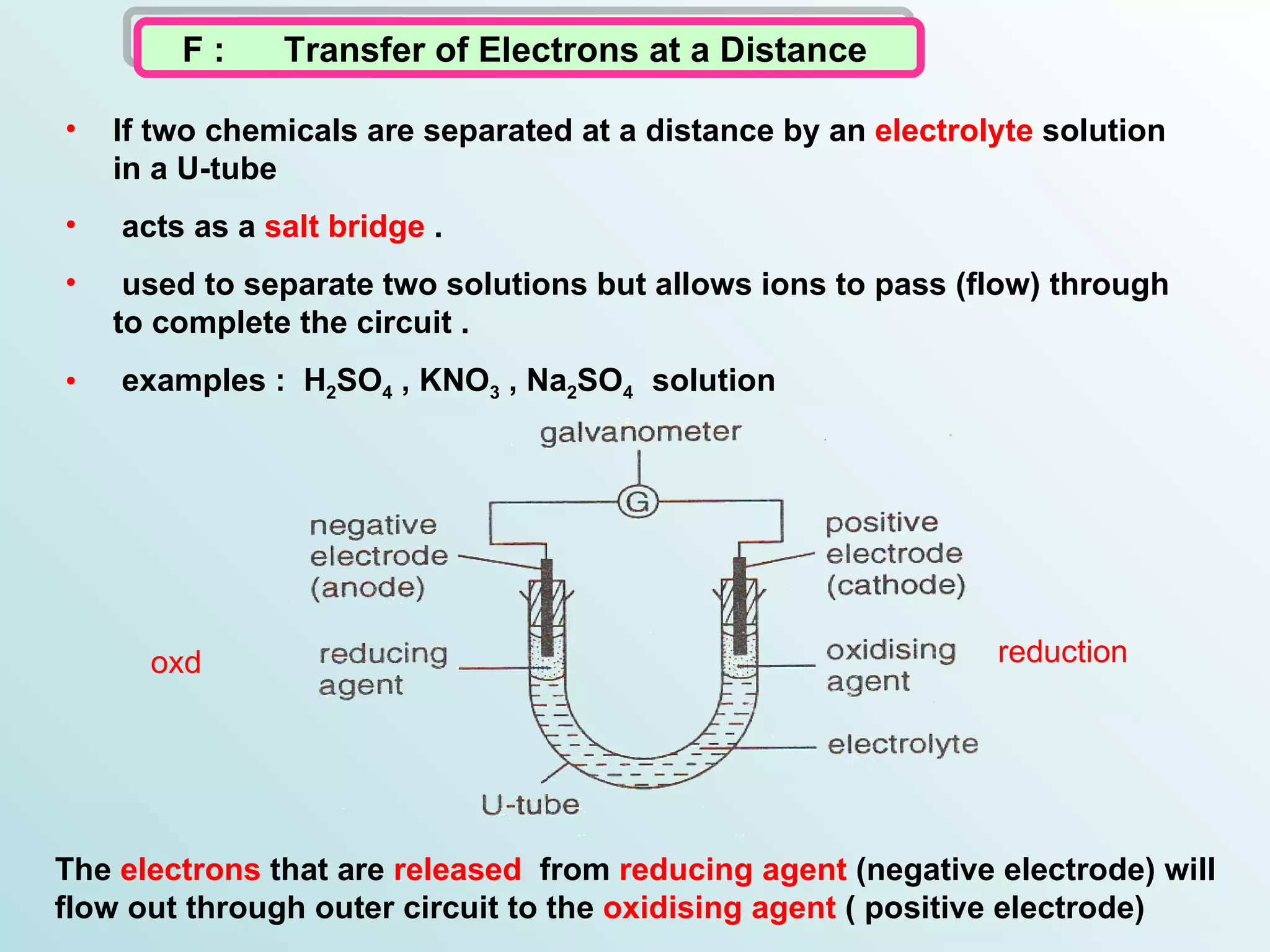
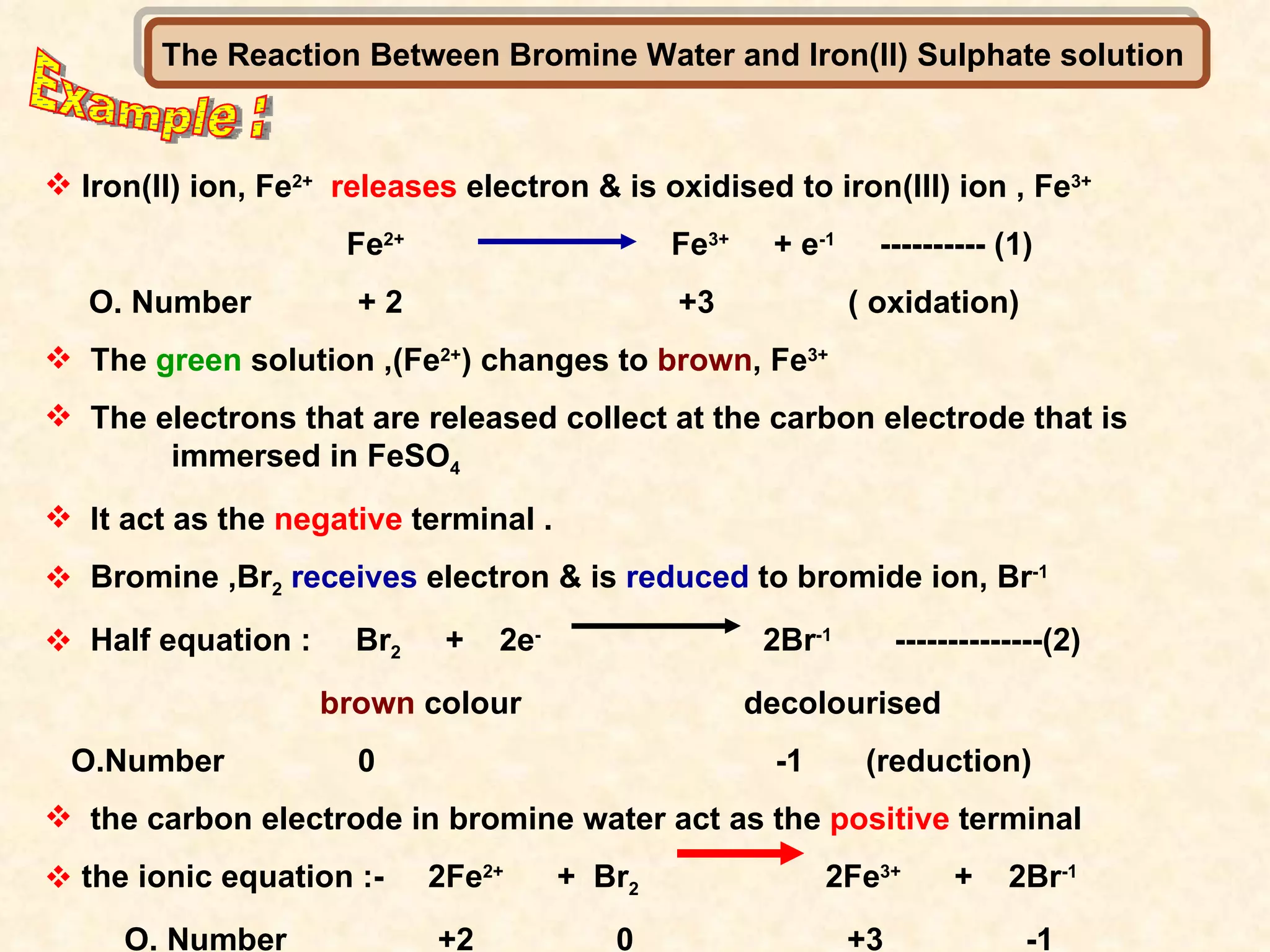
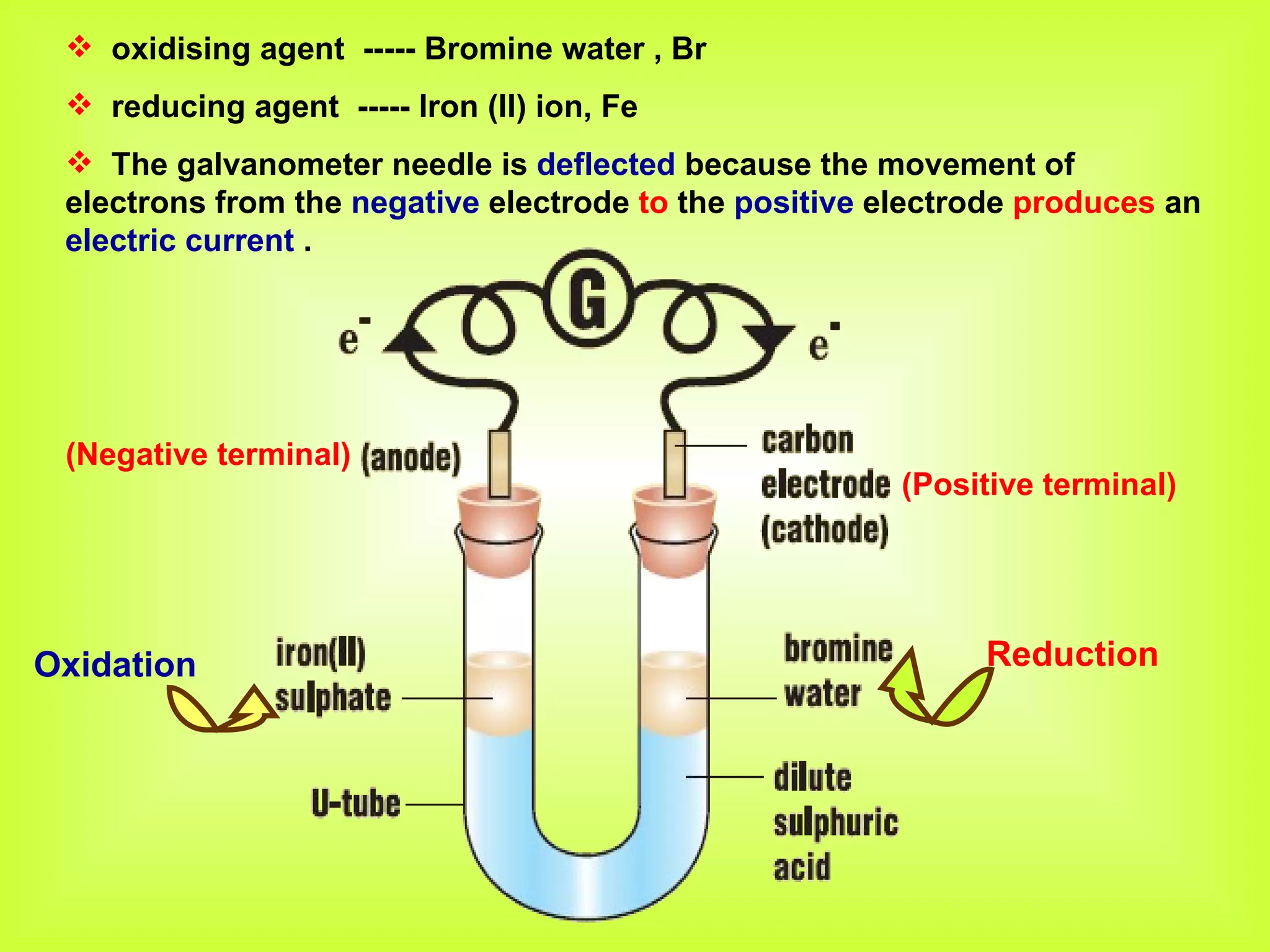
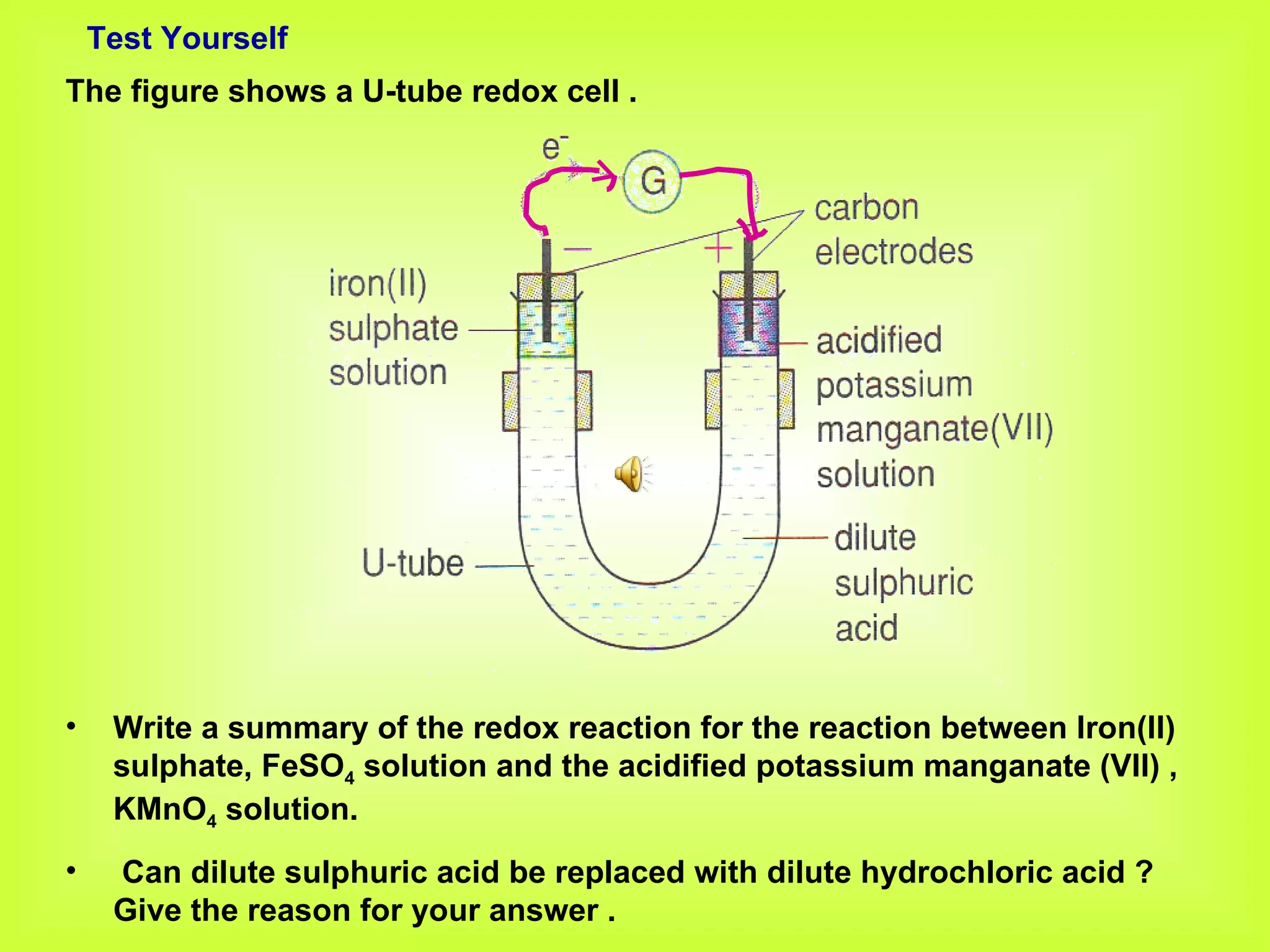
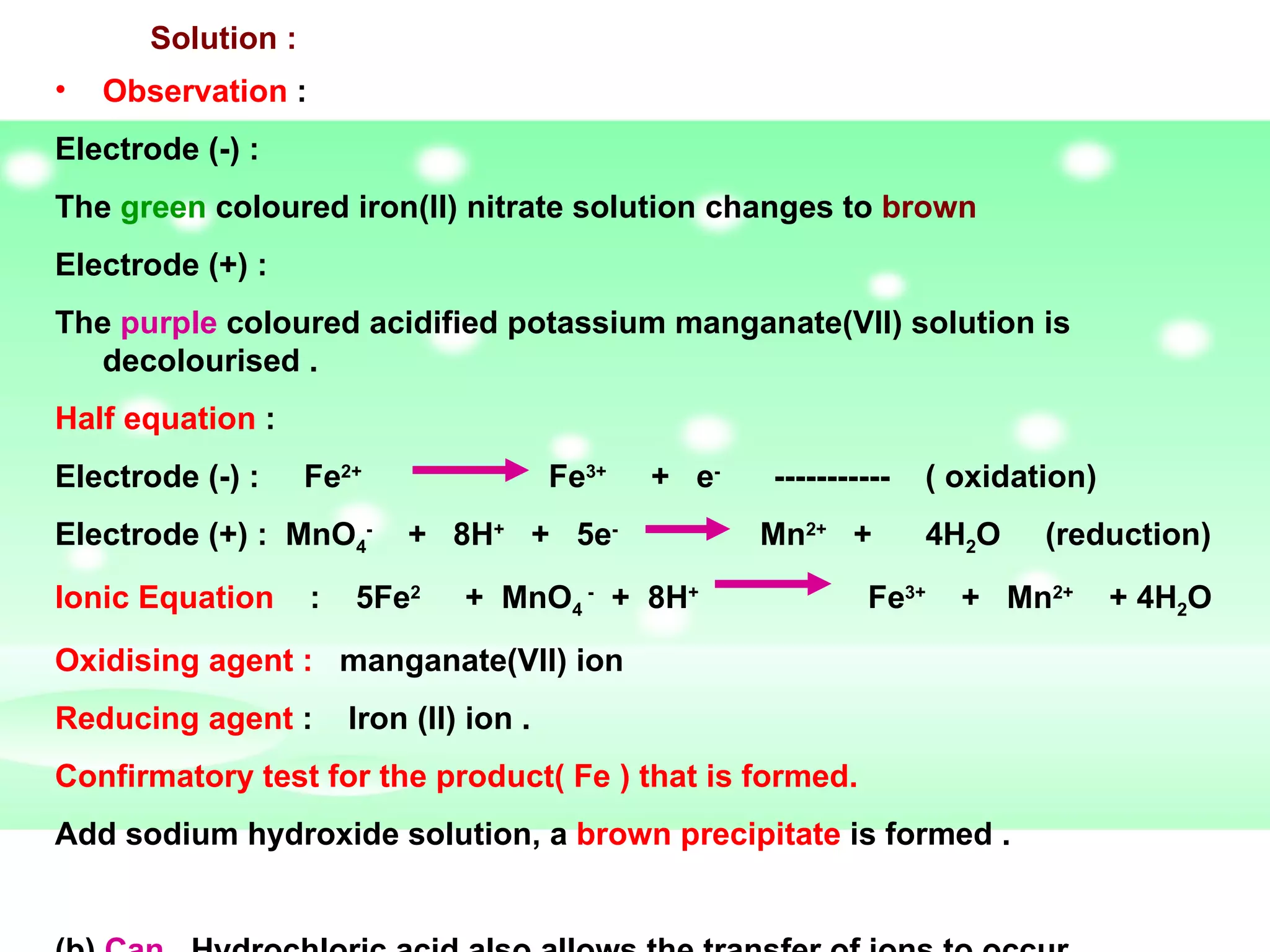
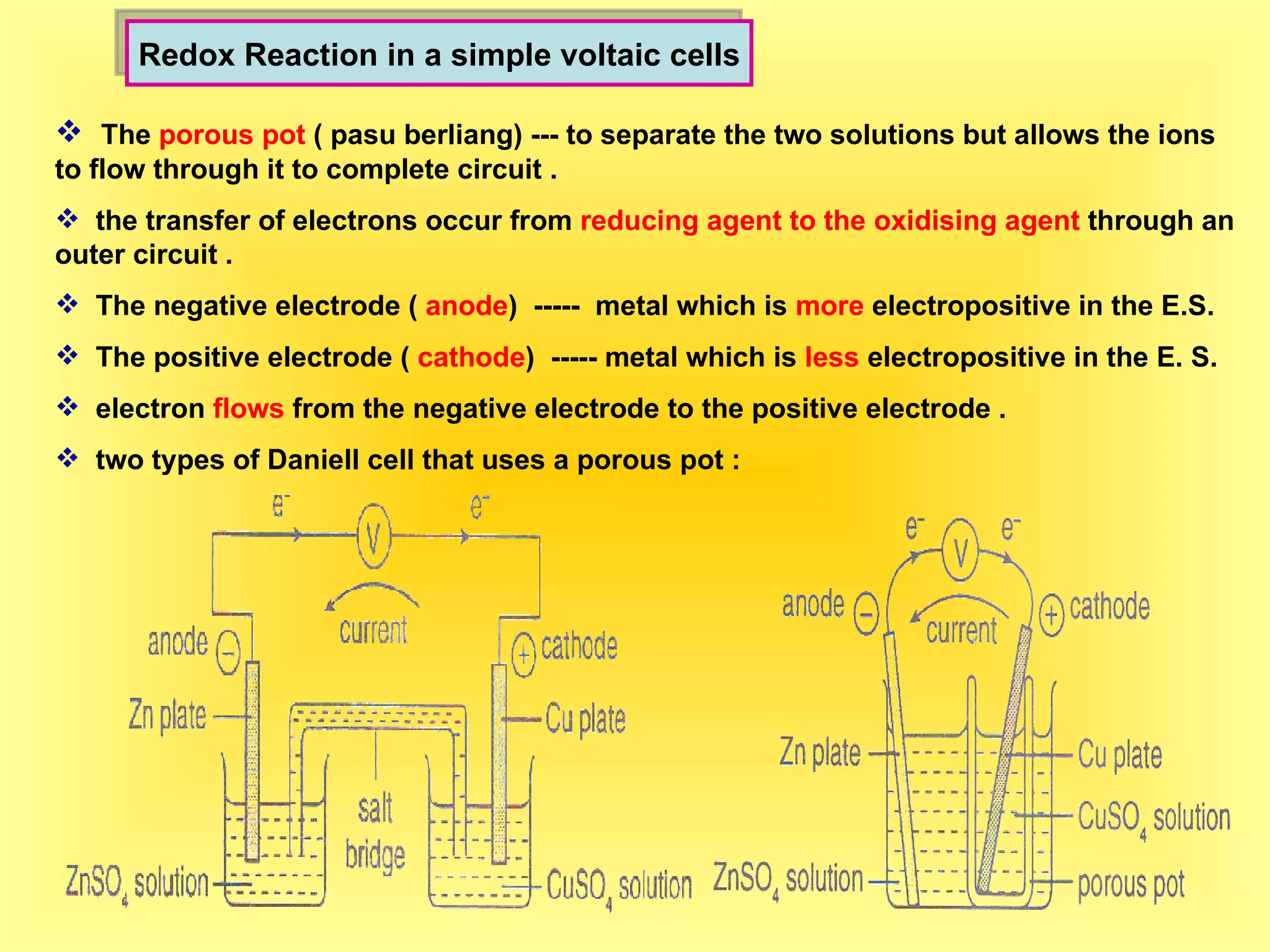
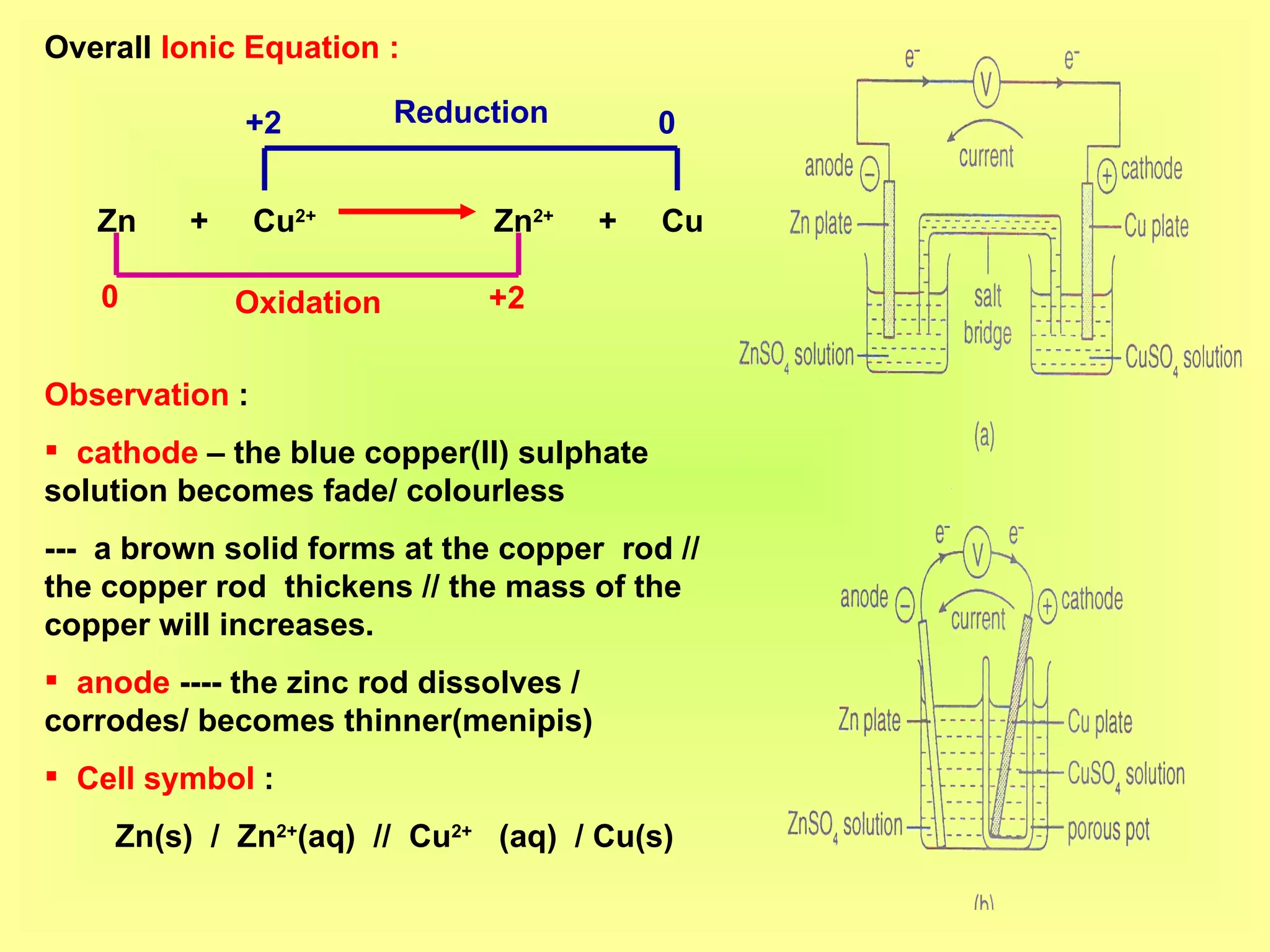
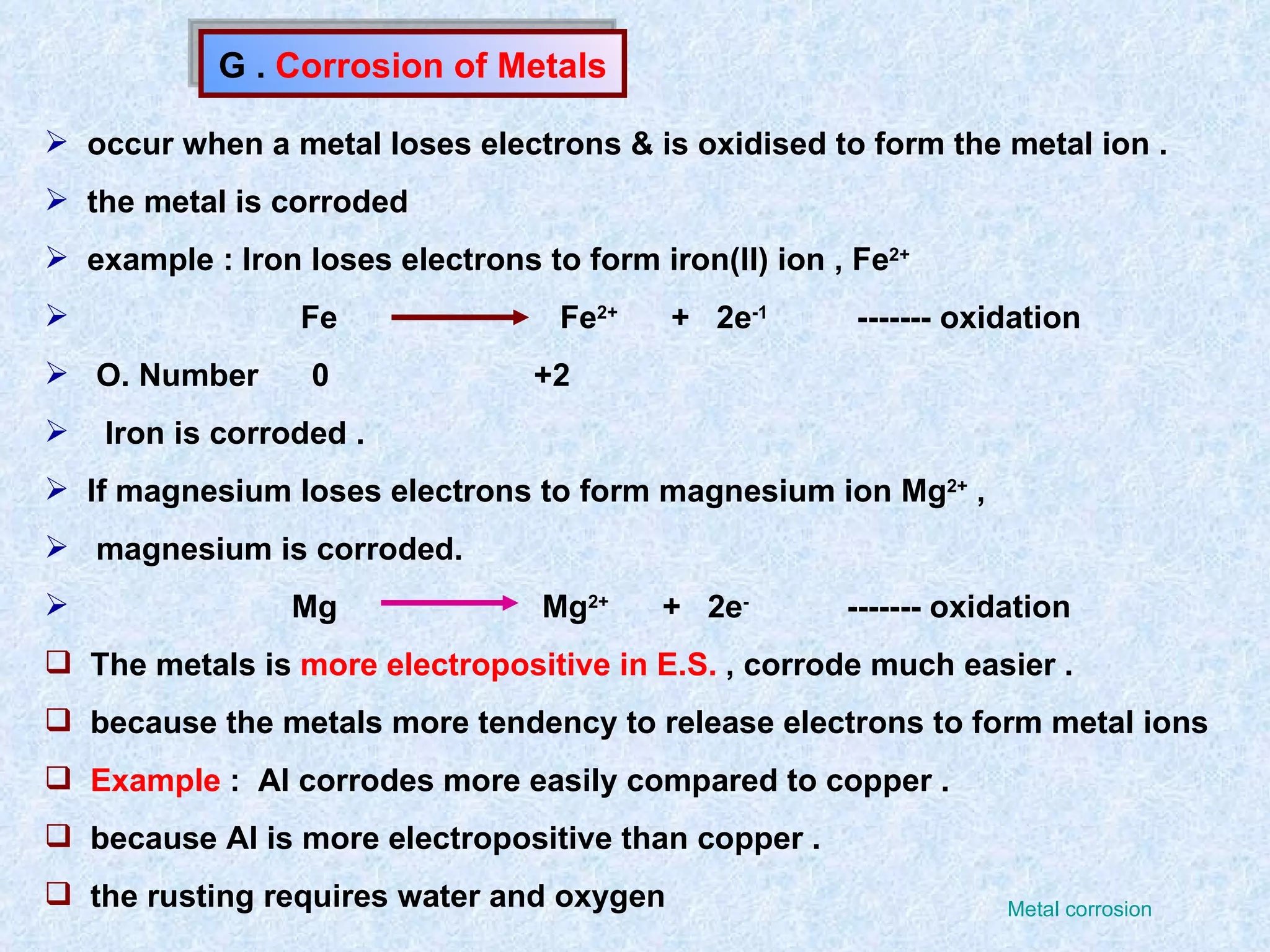
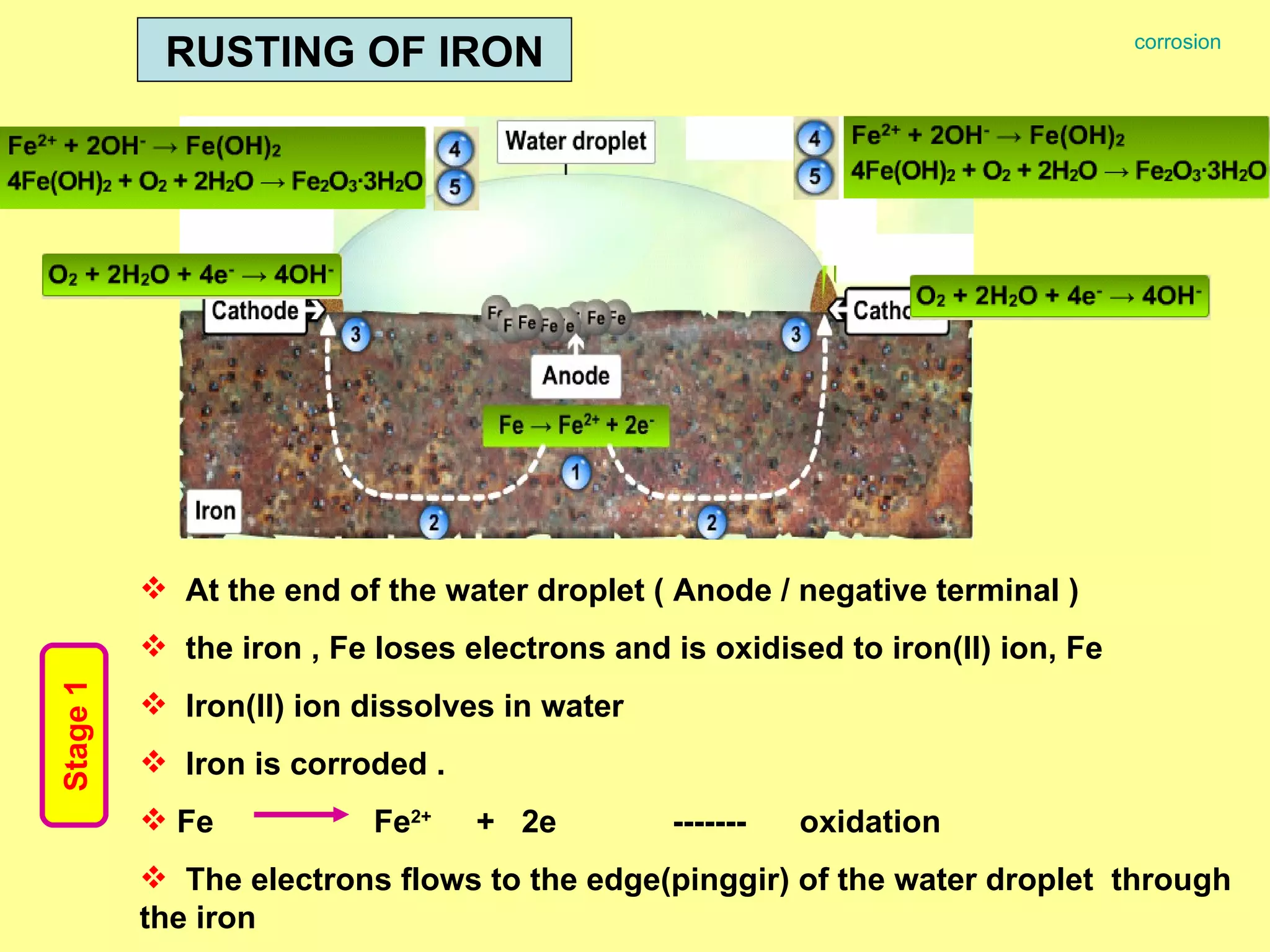
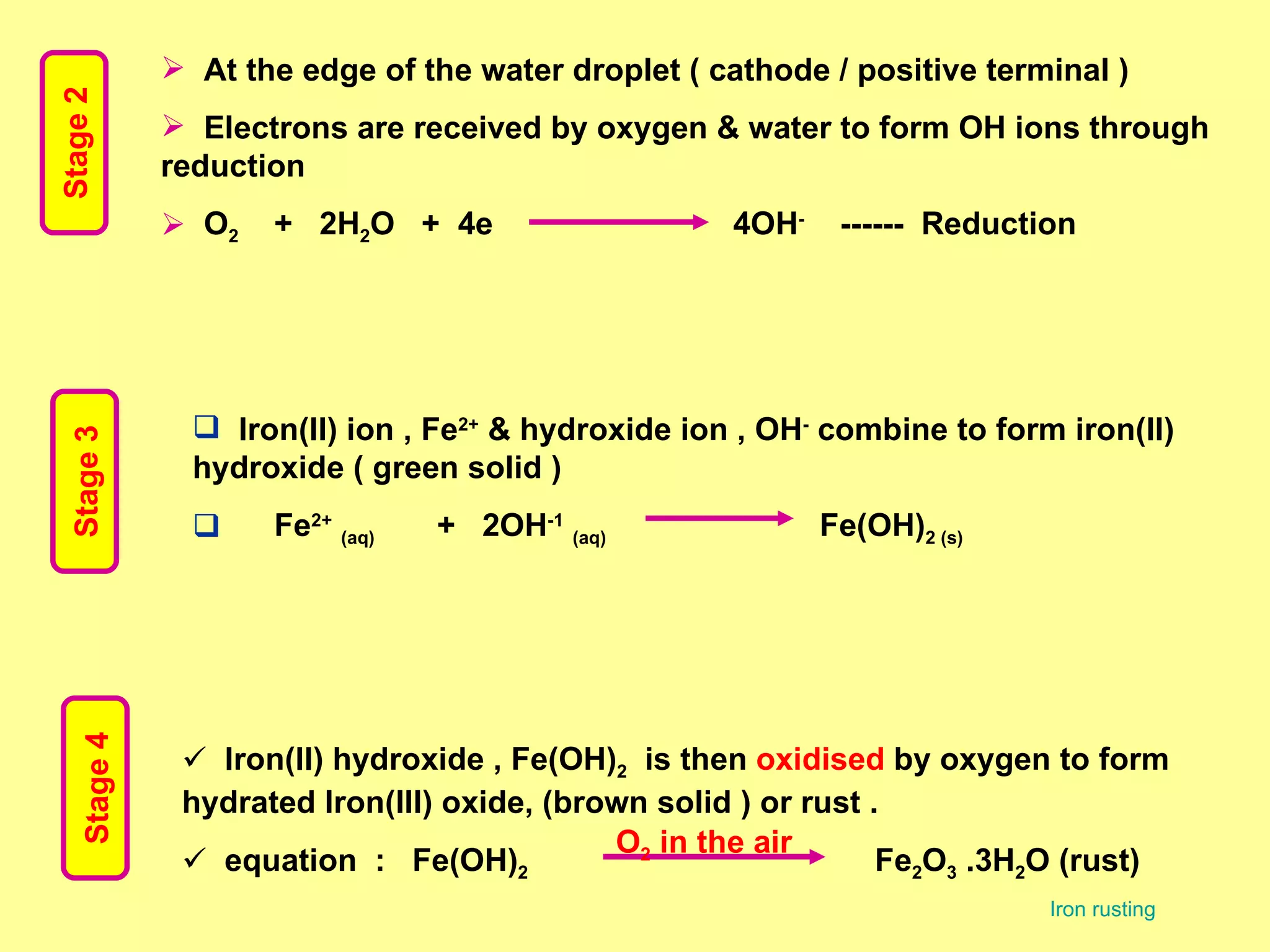
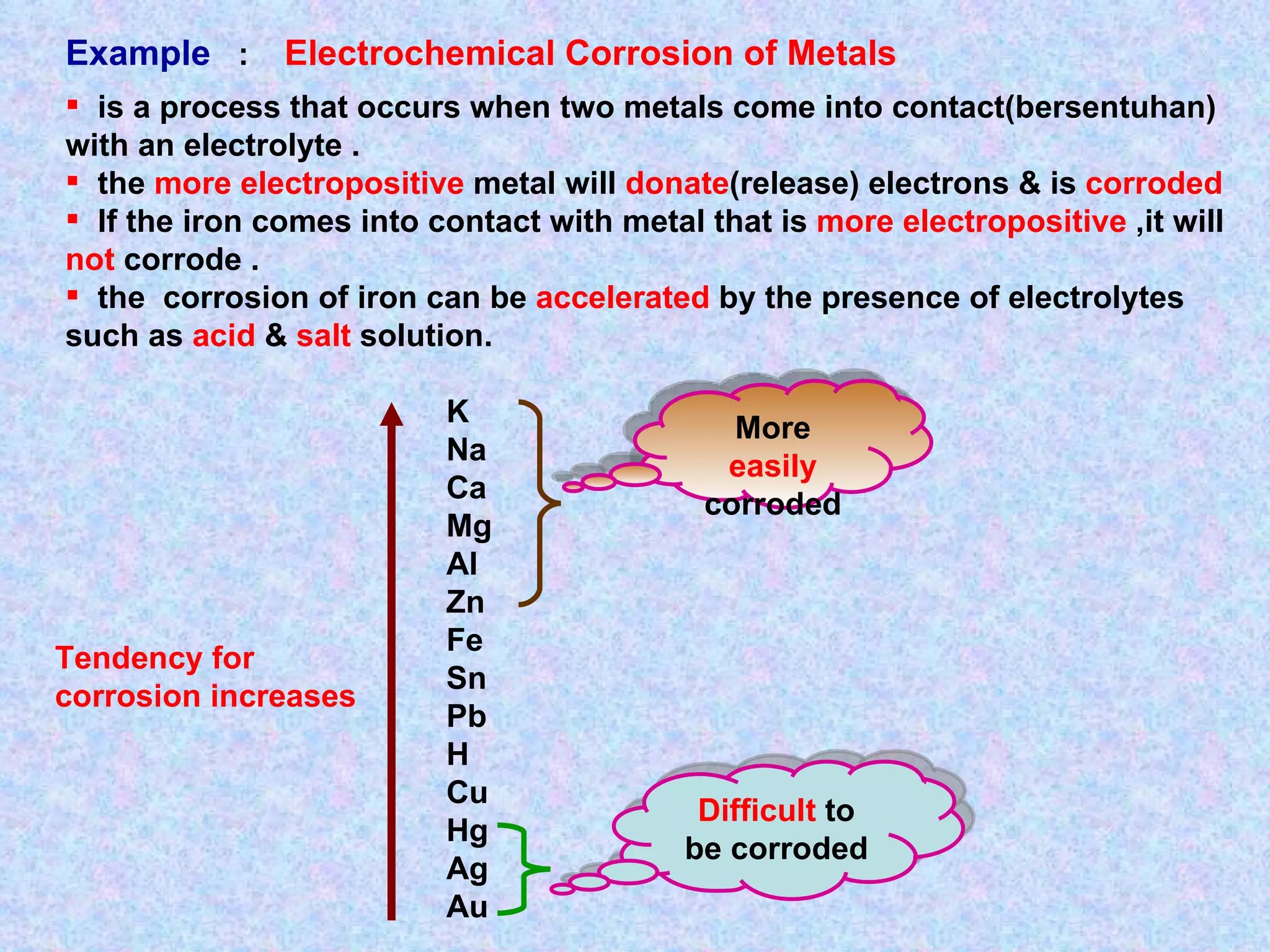
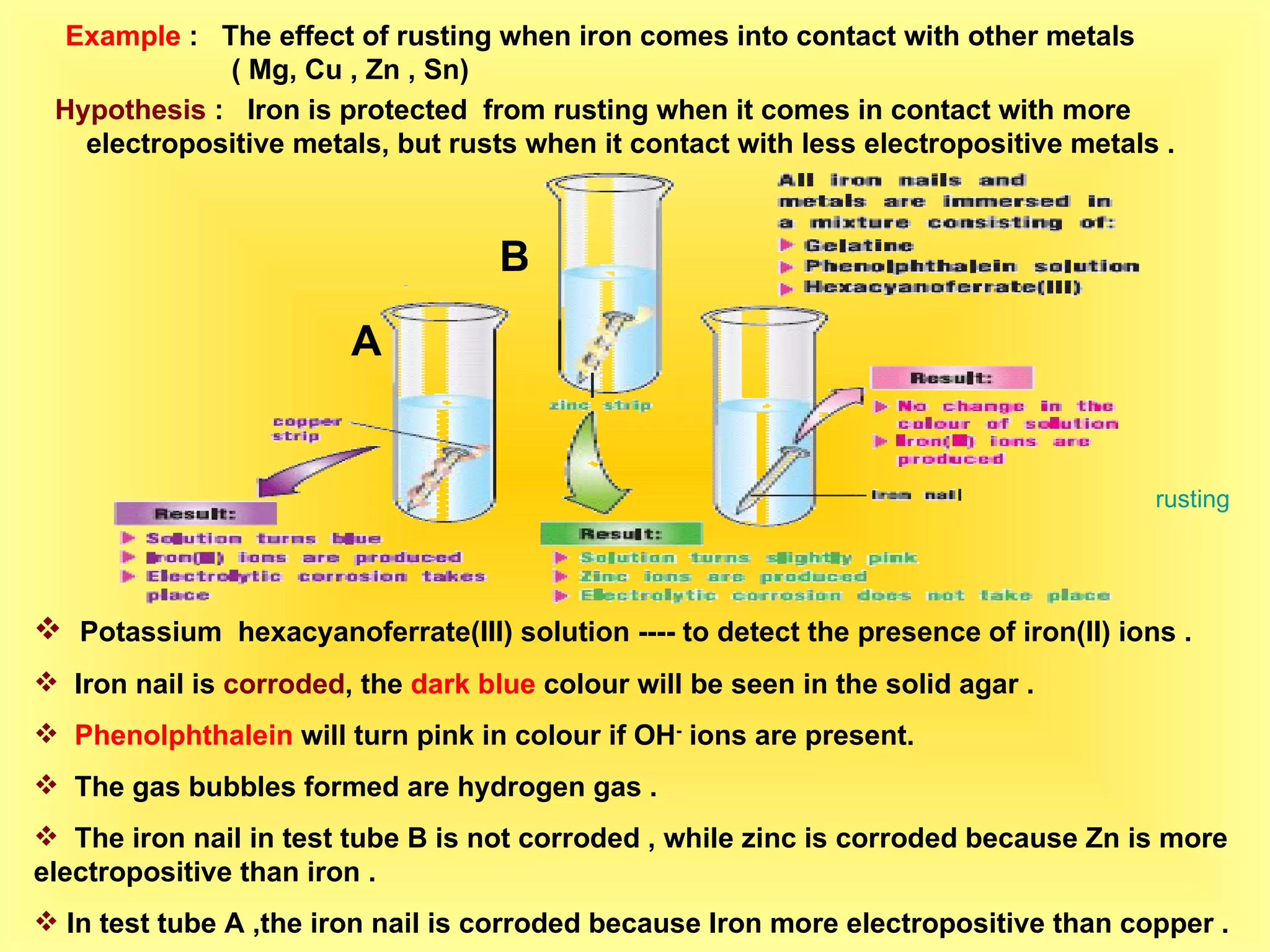
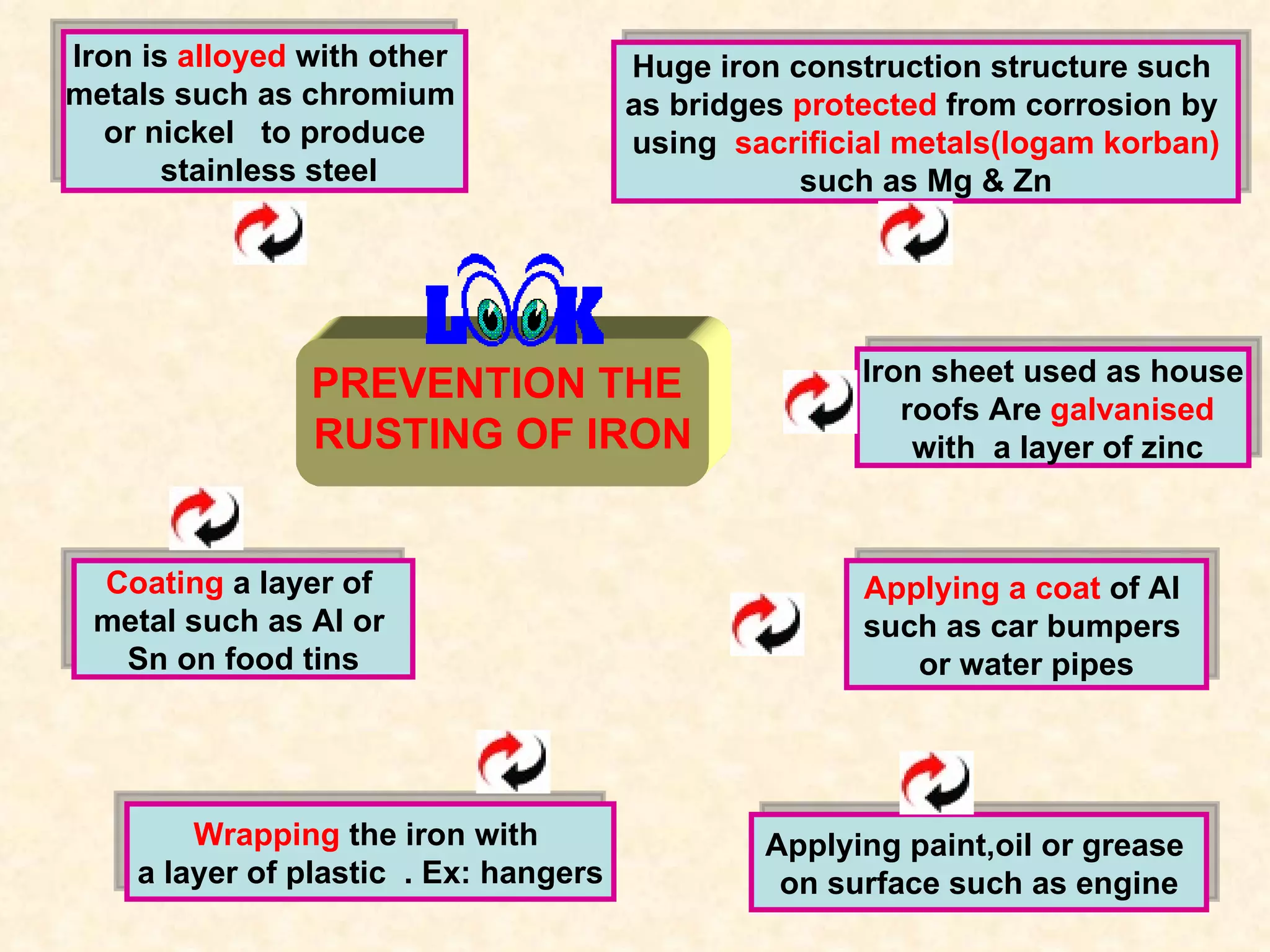
The document discusses redox reactions, including definitions of oxidation, reduction, oxidizing agents and reducing agents. It provides examples of redox reactions like magnesium burning in oxygen, bromine water oxidizing iron(II) ions, and the displacement of metals in salt solutions. It also describes how electrons are transferred during redox reactions and how redox reactions can occur at a distance using a salt bridge.