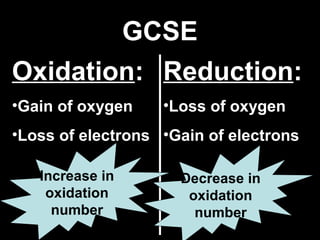
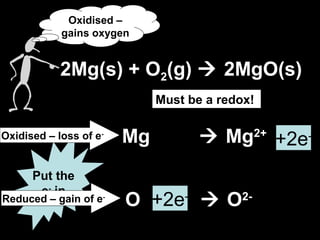
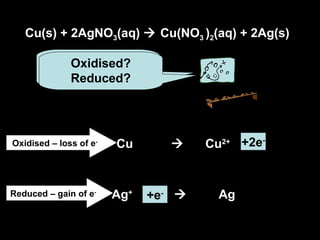
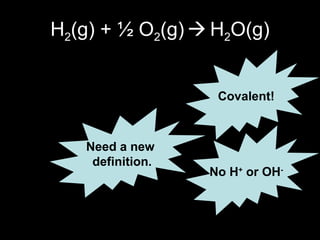
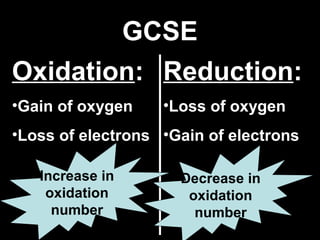
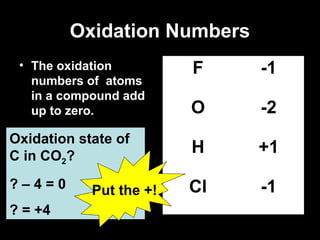
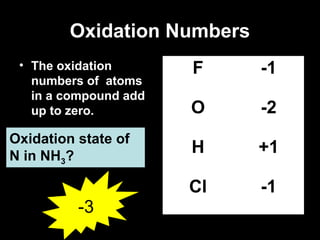
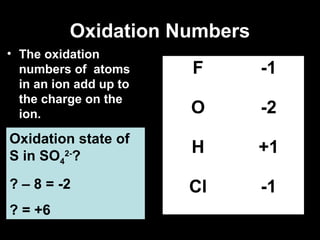
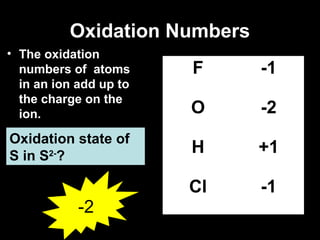
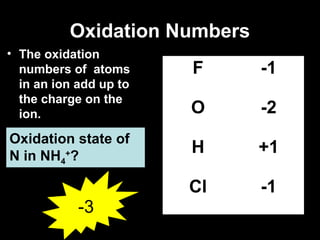
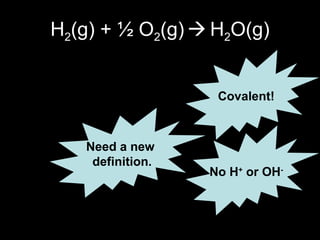
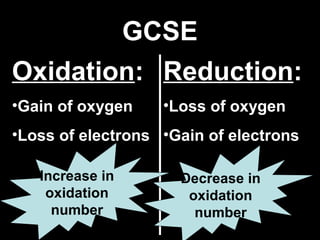
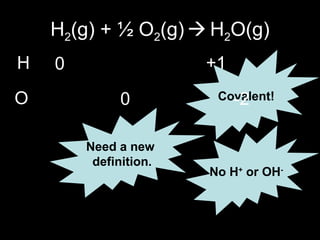
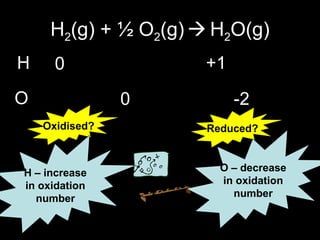
This document discusses redox reactions and oxidation numbers. It defines oxidation as the gain of oxygen or loss of electrons, and reduction as the loss of oxygen or gain of electrons. Examples are given of redox reactions like burning magnesium and copper in silver nitrate solution. Oxidation numbers are introduced as a way to determine if an element is oxidized or reduced in a reaction by calculating the change in electrons. Practice problems are included to help understand how to identify oxidation states and oxidation numbers.