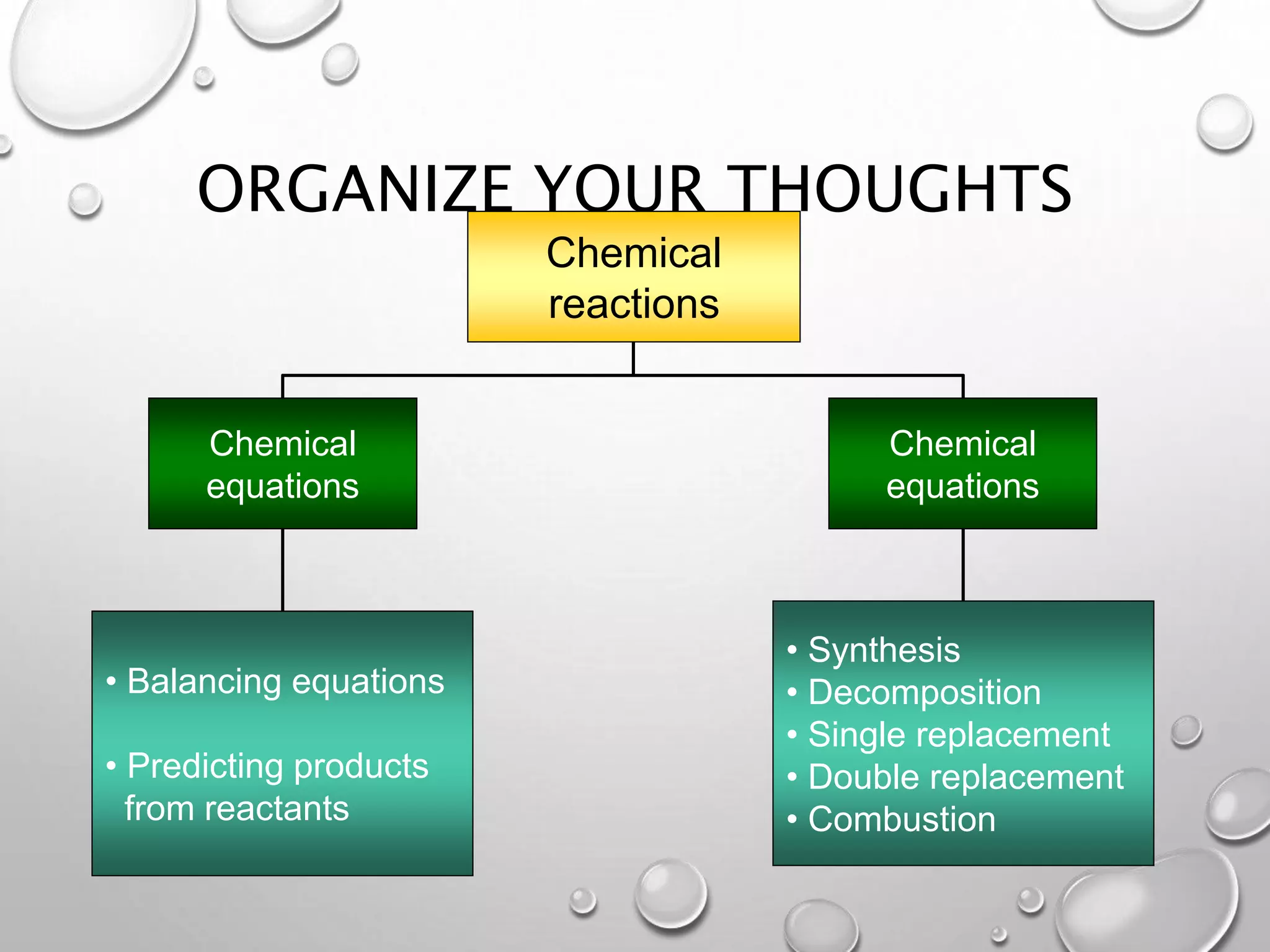
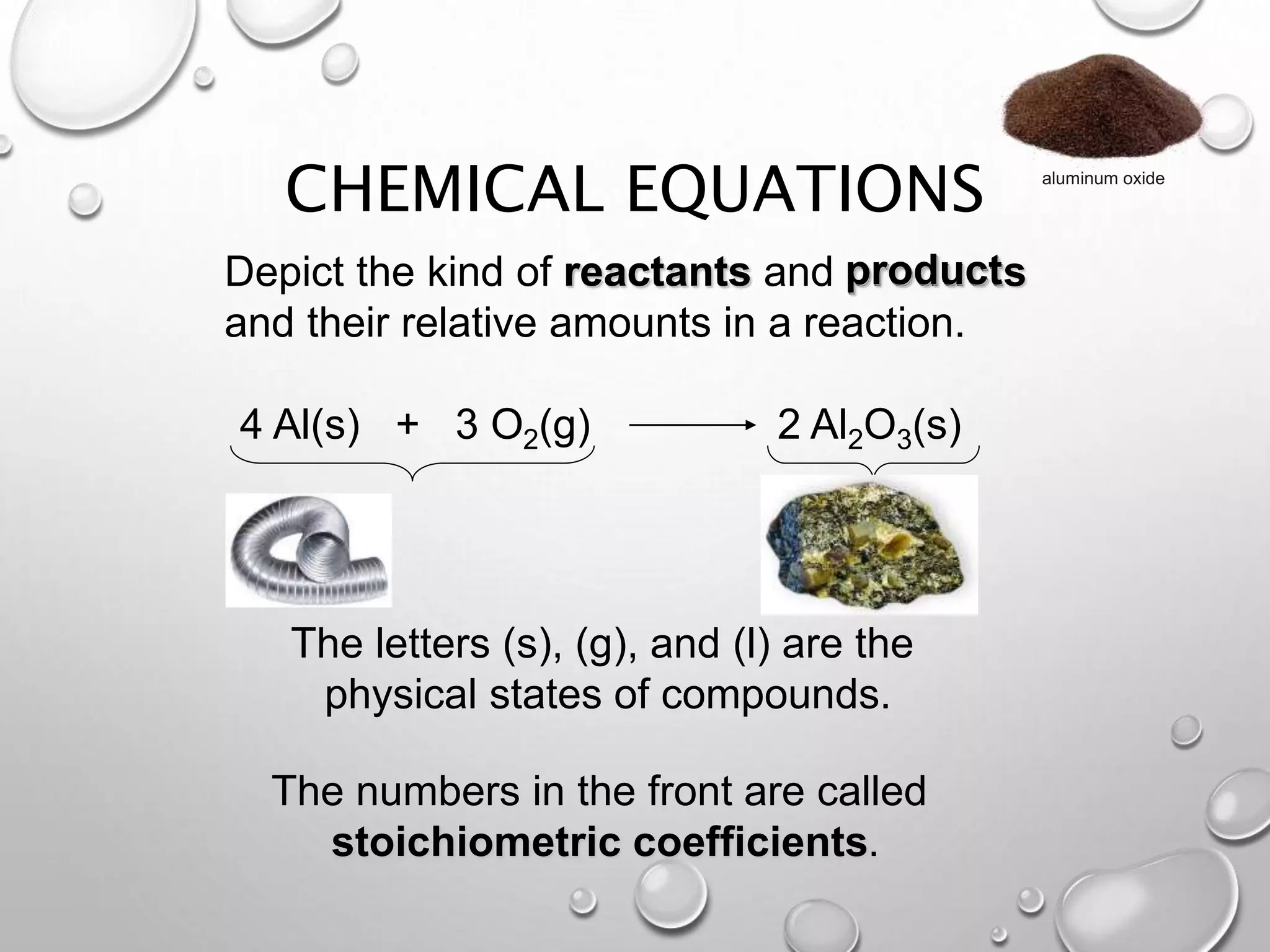
The document discusses chemical equations and reactions. It defines key terms like reactants, products, and coefficients. It explains how to write and balance chemical equations. It also describes different types of chemical reactions like synthesis, decomposition, and single/double replacement reactions. Guidelines are provided for predicting products and writing balanced equations.