This document discusses redox reactions and oxidation numbers:
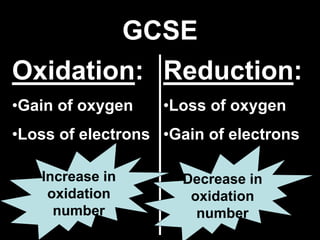
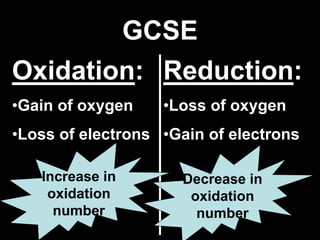
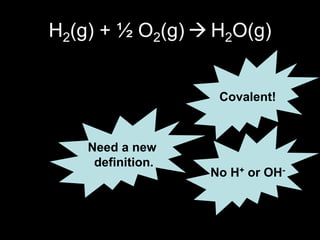
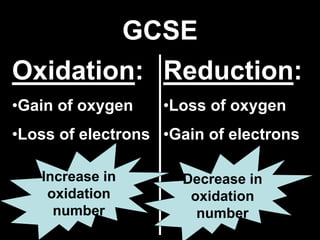
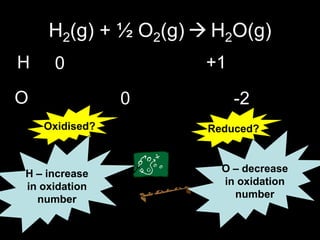
1. It defines oxidation as the gain of oxygen or loss of electrons, and reduction as the loss of oxygen or gain of electrons. Both are characterized by changes in oxidation number.
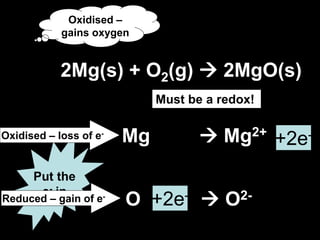
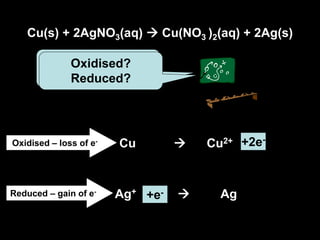
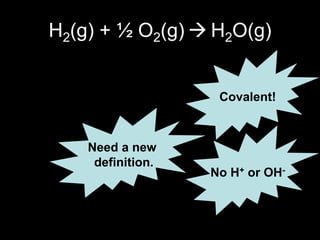
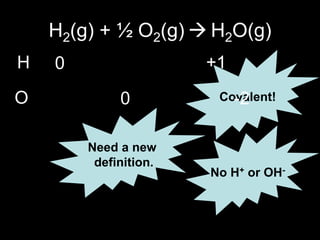
2. Four experiments are described that demonstrate redox reactions: burning magnesium, copper in silver nitrate solution, chlorine and potassium iodide solution, and exploding hydrogen.
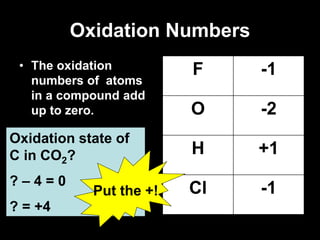
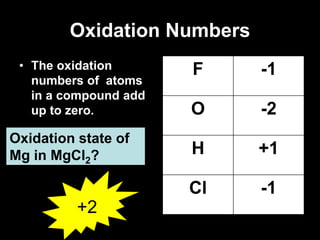
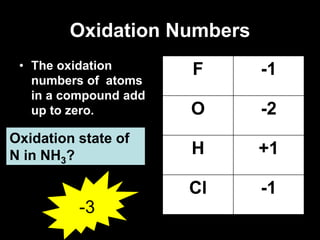
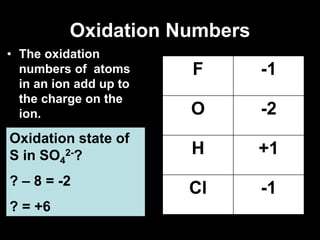
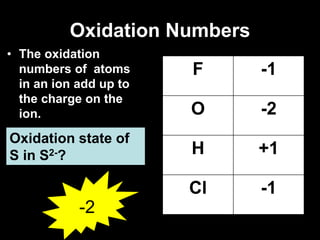
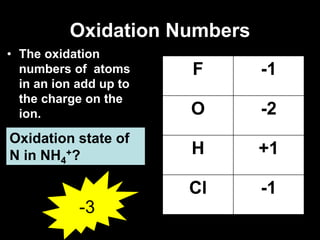
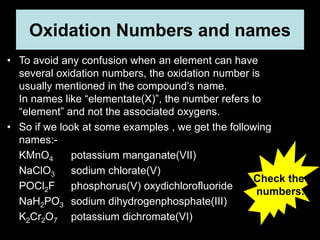
3. Oxidation numbers are assigned according to rules such as elements in their natural state having an oxidation number of 0 and the oxidation numbers of compounds summing to the overall charge. Examples of assigning oxidation numbers are provided.