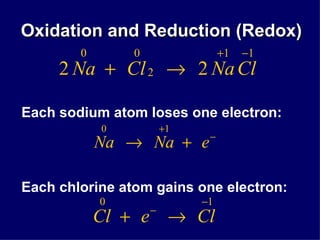
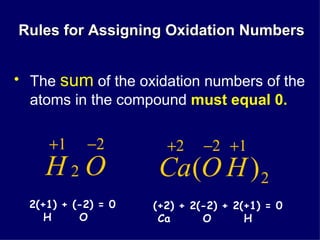
The document discusses oxidation-reduction (redox) reactions. It defines oxidation as the loss of electrons and reduction as the gain of electrons. Redox reactions always involve both an oxidation and a reduction process. The substance undergoing oxidation is the reducing agent as it loses electrons, while the substance undergoing reduction is the oxidizing agent as it gains electrons. Assigning oxidation numbers to atoms allows identification of which substances are oxidized and reduced in a reaction.