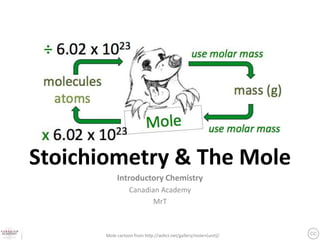
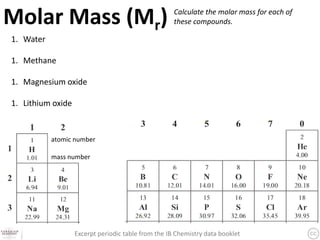
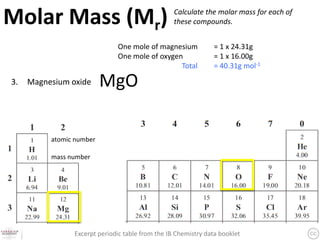
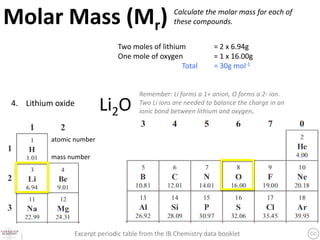
The document discusses the mole and Avogadro's number (6.02 x 10^23) as a way to quantify large numbers of particles, such as atoms and molecules. It explains how one mole of a substance contains 6.02 x 10^23 units, similar to a dozen representing 12 items. Additionally, it covers practical applications of molar solutions in chemistry, including how to prepare specific concentrations of solute in water.