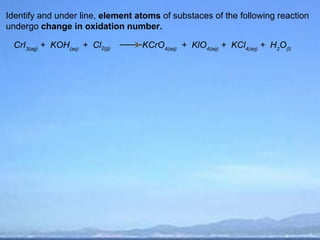
The document discusses redox (reduction-oxidation) reactions. It defines oxidation and reduction in terms of electron transfer and changes in oxidation number. Key points include:
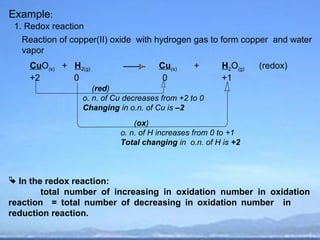
1) Redox reactions involve the transfer of electrons from a reducing agent to an oxidizing agent.
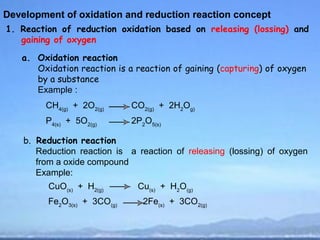
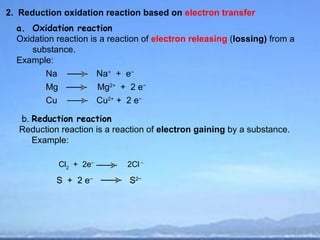
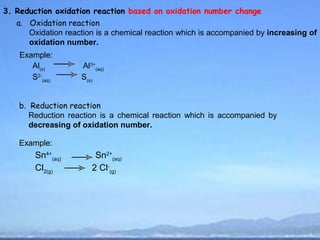
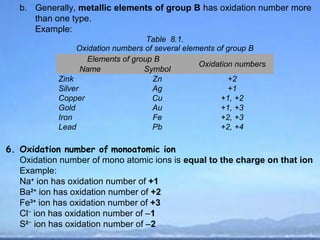
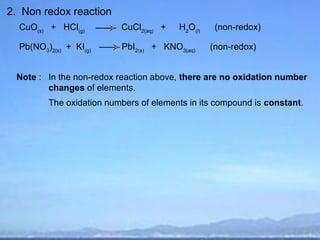
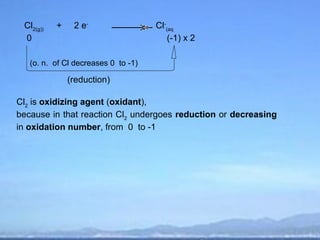
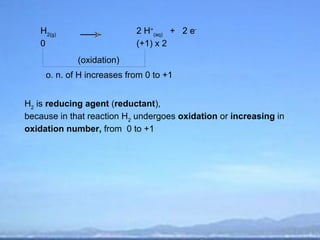
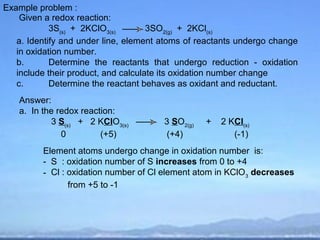
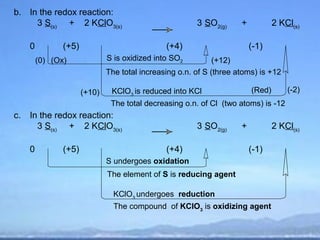
2) Oxidation is the loss of electrons or an increase in oxidation number, while reduction is the gain of electrons or a decrease in oxidation number.
3) Oxidizing agents undergo reduction by gaining electrons, while reducing agents undergo oxidation by losing electrons.

![REDUCTION AND OXIDATION REACTION
Development of oxidation and reduction reaction concept
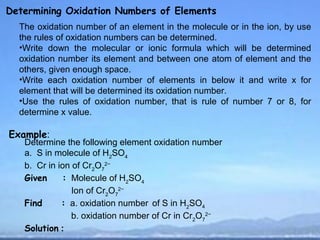
Oxidation Numbers
Determining Oxidation Numbers of Elements
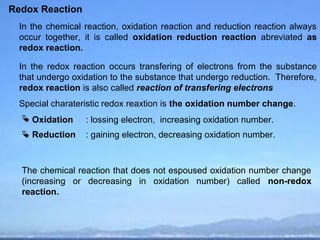
Redox Reaction
Oxidizing Agent [Oxidant] and Reducing Agent [Reductant]
Auto Redox Reaction
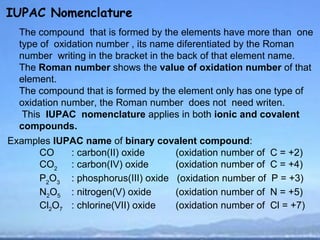
IUPAC Nomenclature](https://image.slidesharecdn.com/redoxreaction-120912083250-phpapp02/85/Redox-reaction-2-320.jpg)