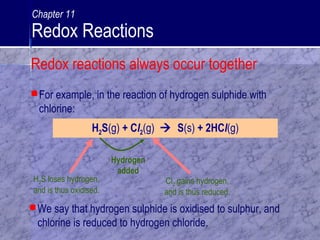
The document defines oxidation and reduction as well as oxidizing and reducing agents. It explains that oxidation is the loss of electrons or gain of oxygen, while reduction is the gain of electrons or loss of oxygen. Oxidizing agents cause oxidation by accepting electrons, while reducing agents cause reduction by donating electrons. Redox reactions involve both oxidation and reduction halves that always occur together. Tests are described to identify oxidizing agents using potassium iodide solution, which will turn reddish brown if iodine is liberated, indicating an oxidizing agent is present.