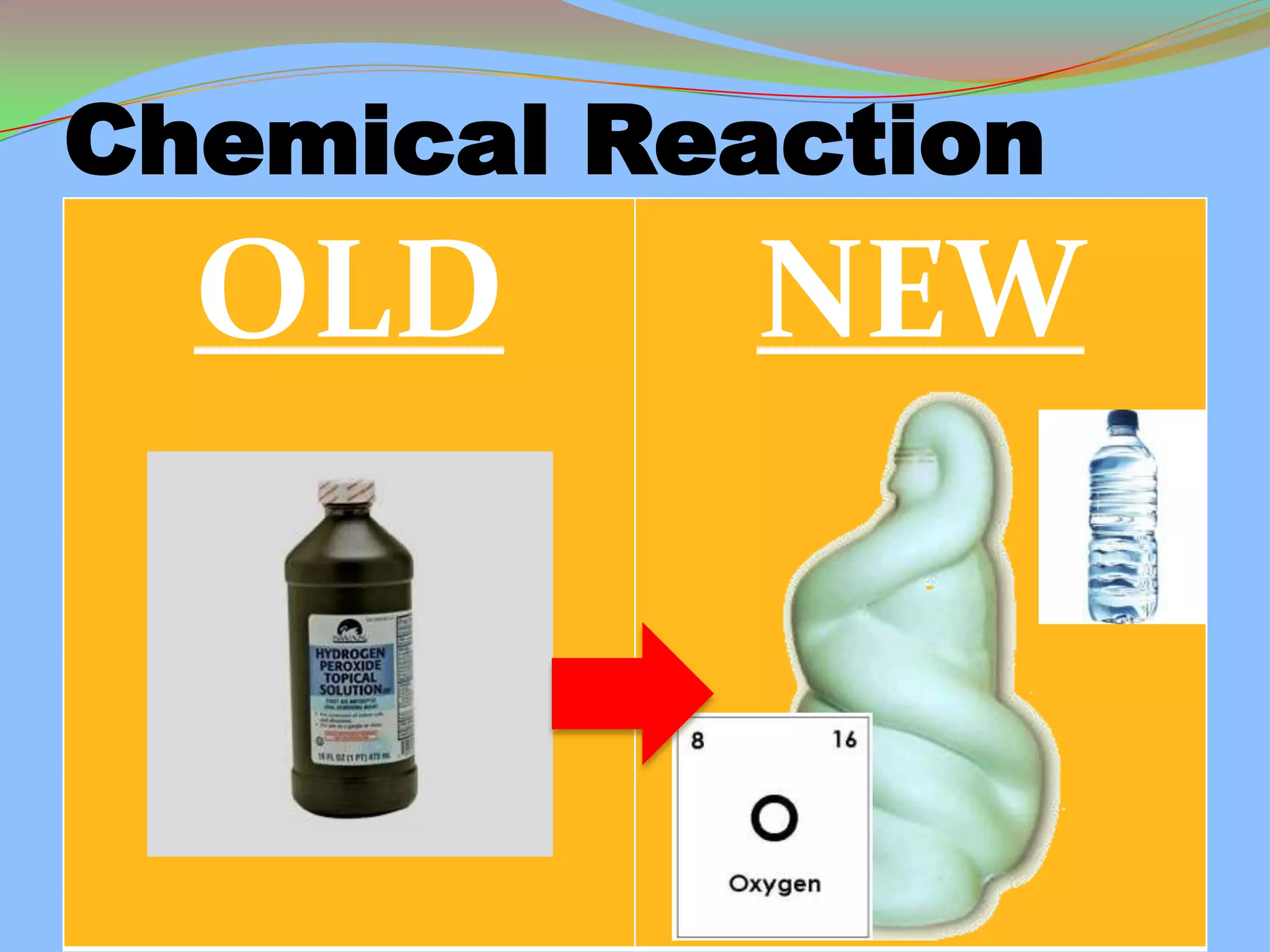
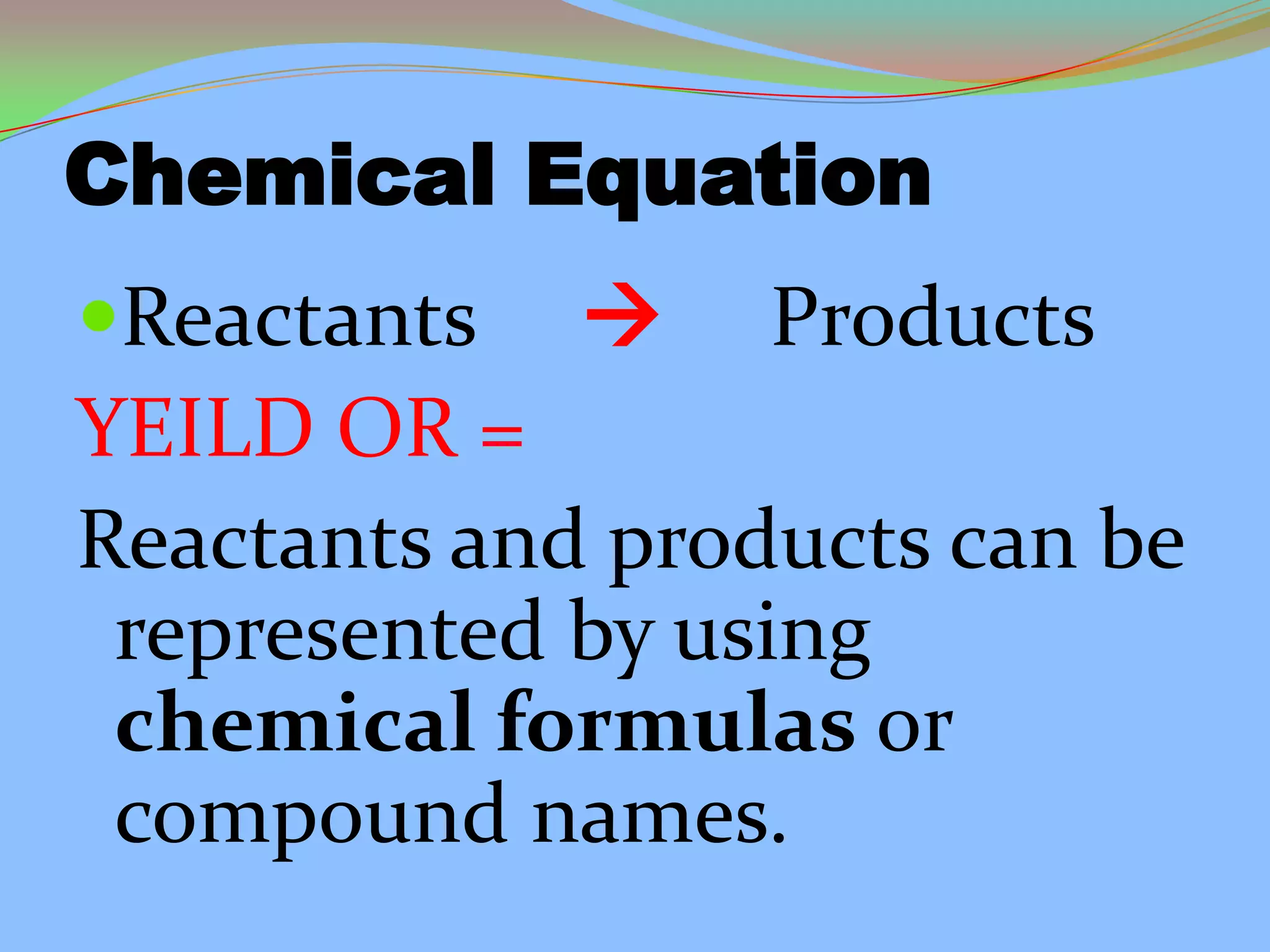
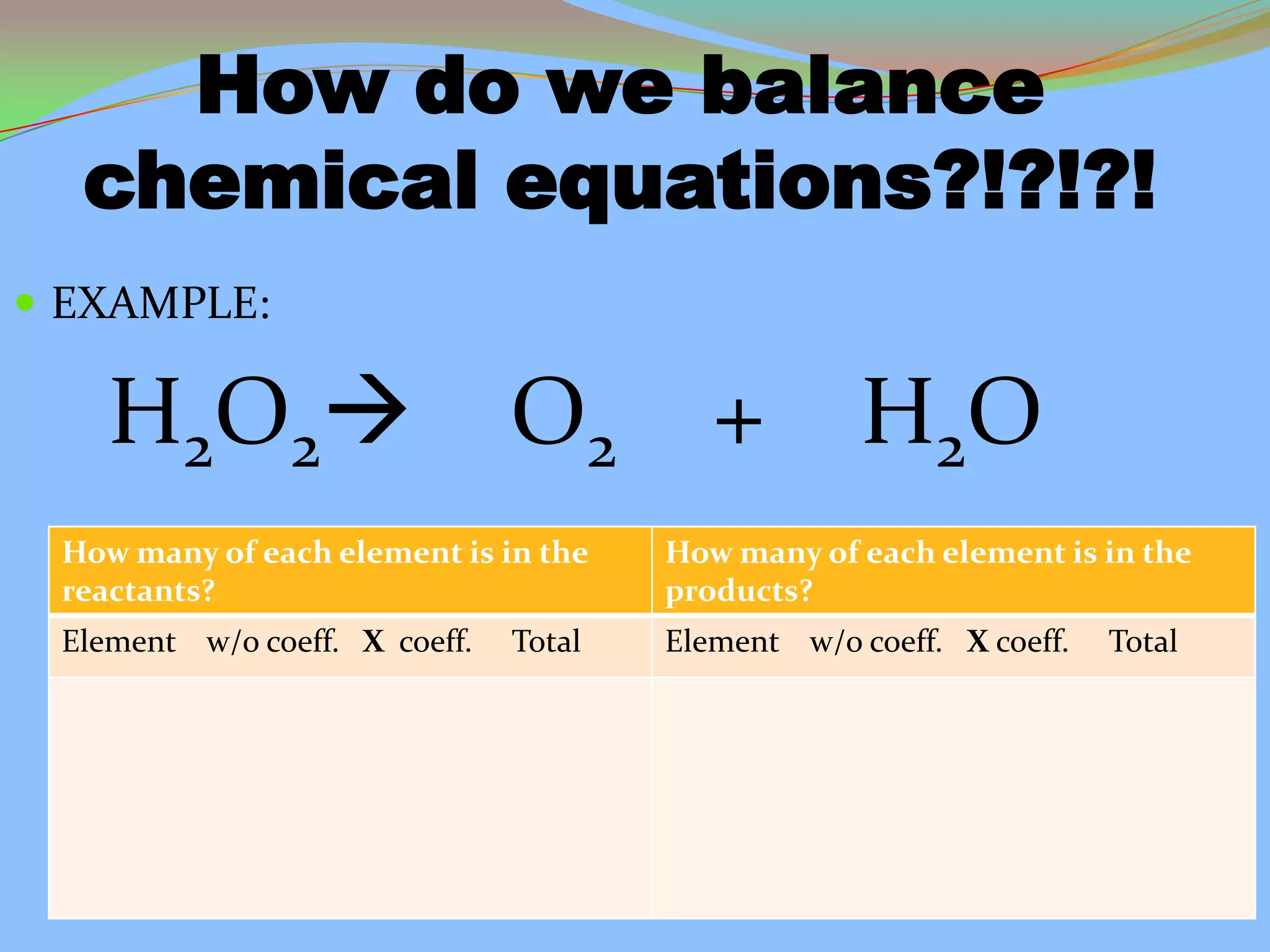
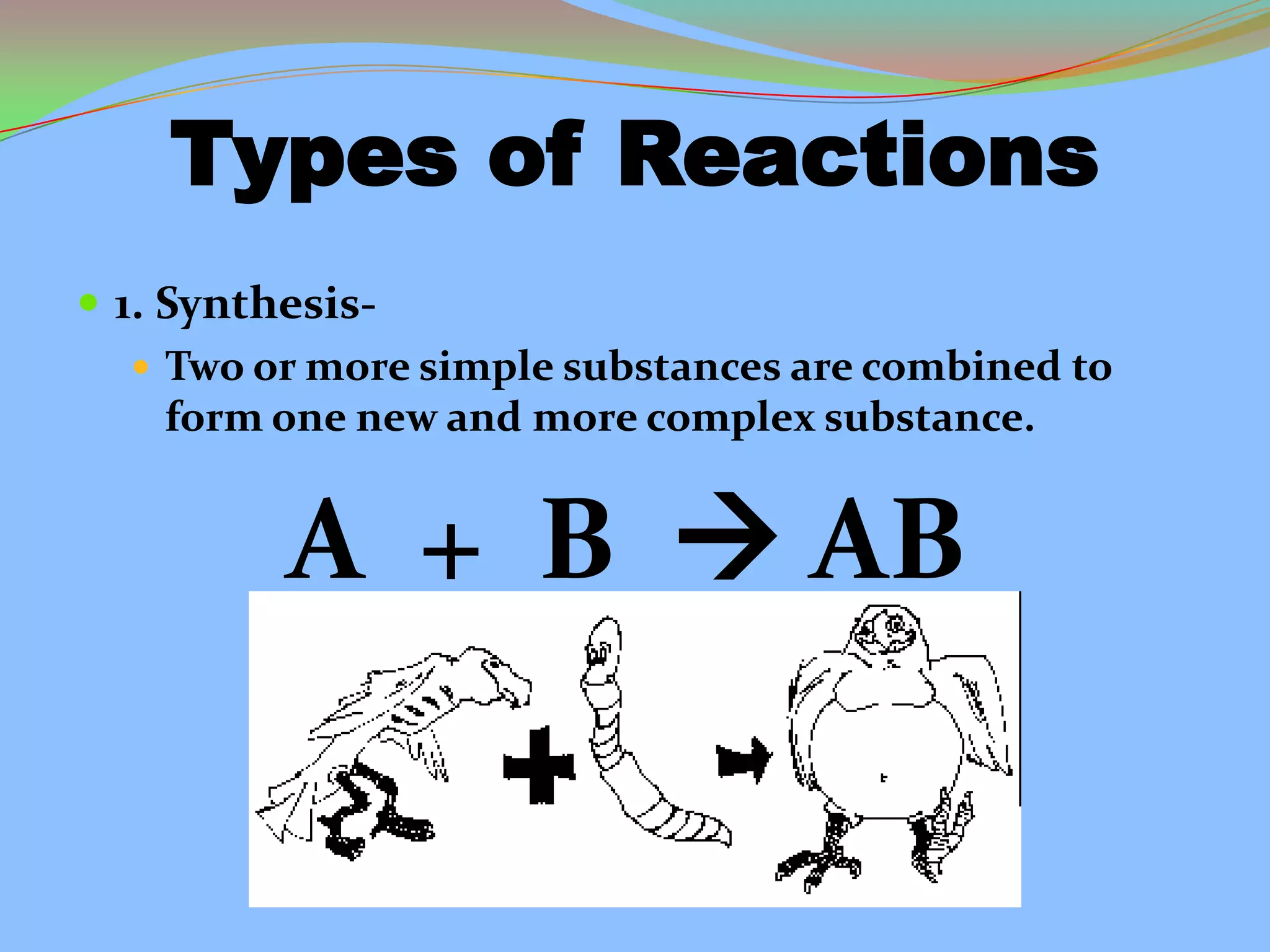
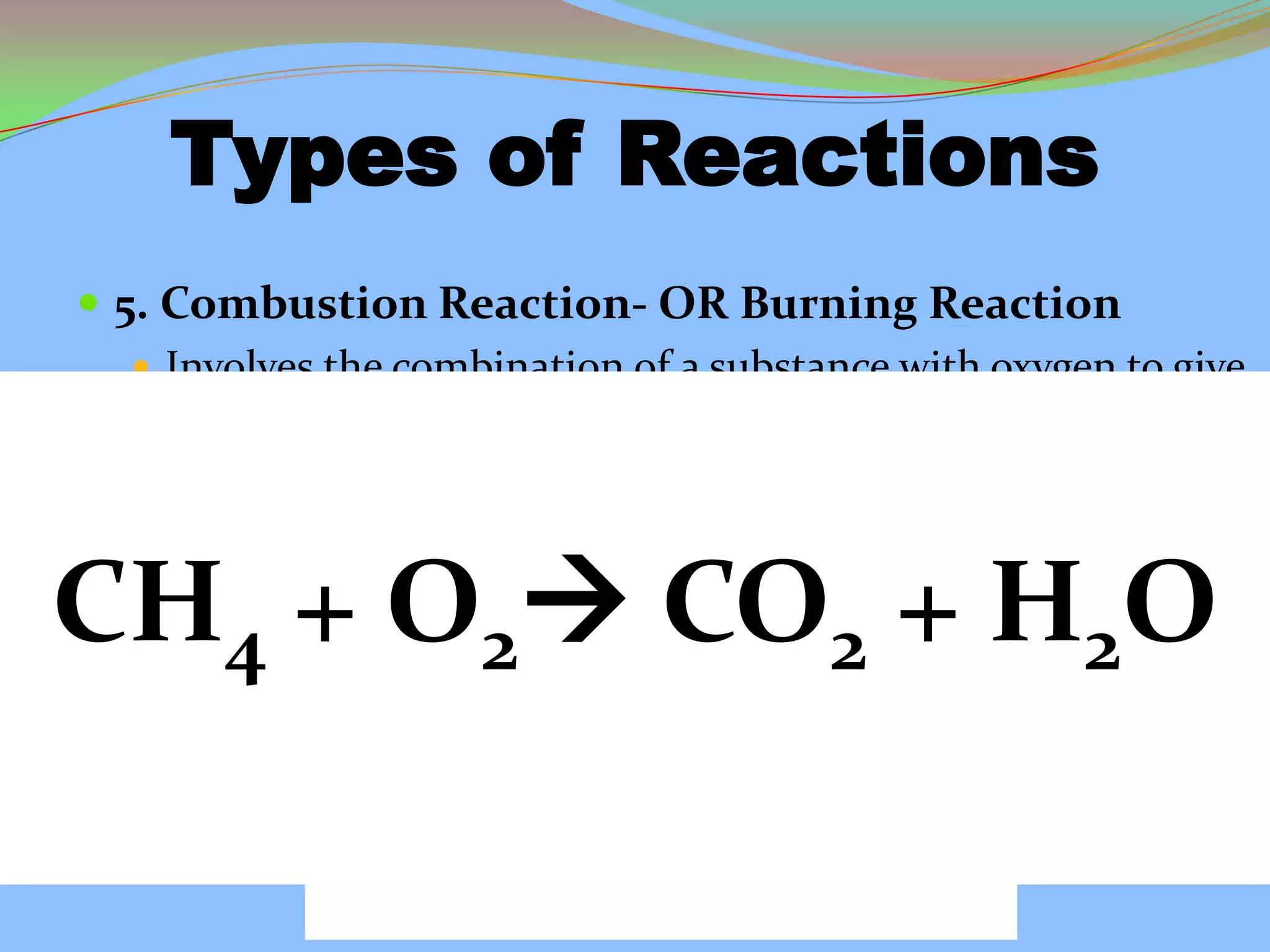
This document defines and describes chemical reactions. It explains that in a chemical reaction, atoms rearrange to form new compounds, with old bonds breaking and new bonds forming. Chemical reactions can be represented by chemical equations that show the reactants and products. Reactions can be exothermic, releasing energy, or endothermic, absorbing energy. They can also vary in reaction rate. The document also covers the law of conservation of matter, balancing chemical equations, and the main types of chemical reactions: synthesis, decomposition, single replacement, double replacement, and combustion reactions.