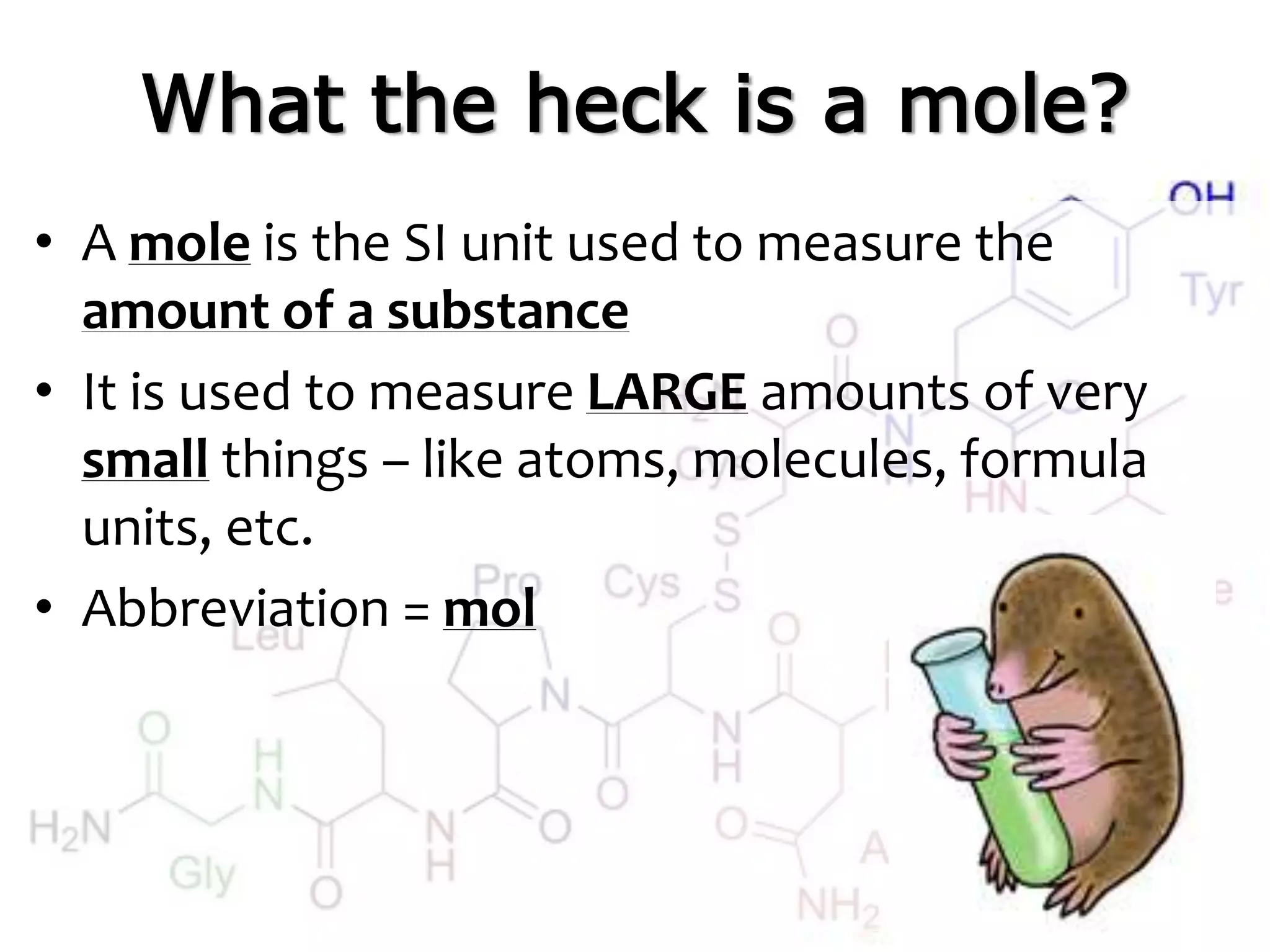
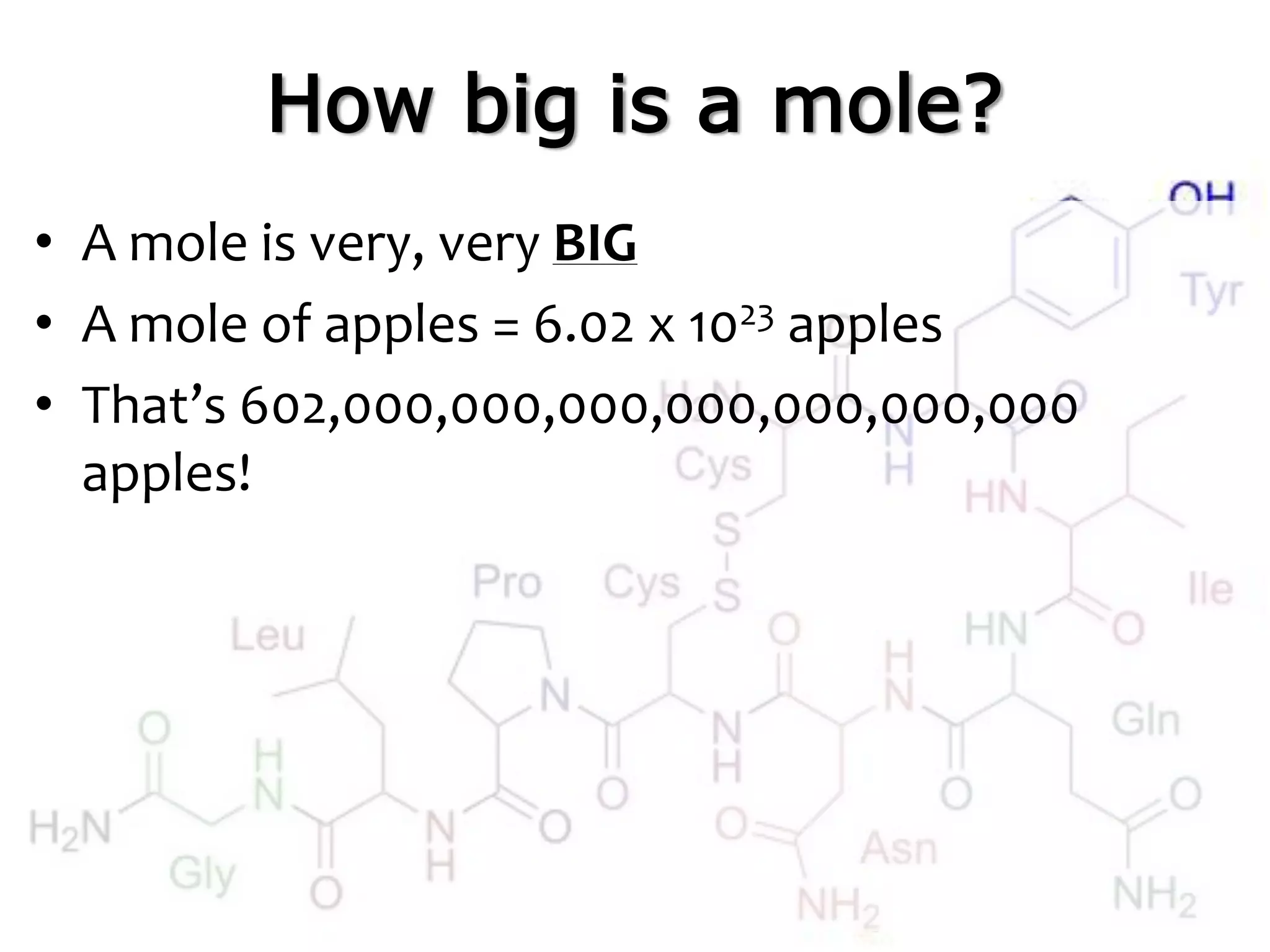
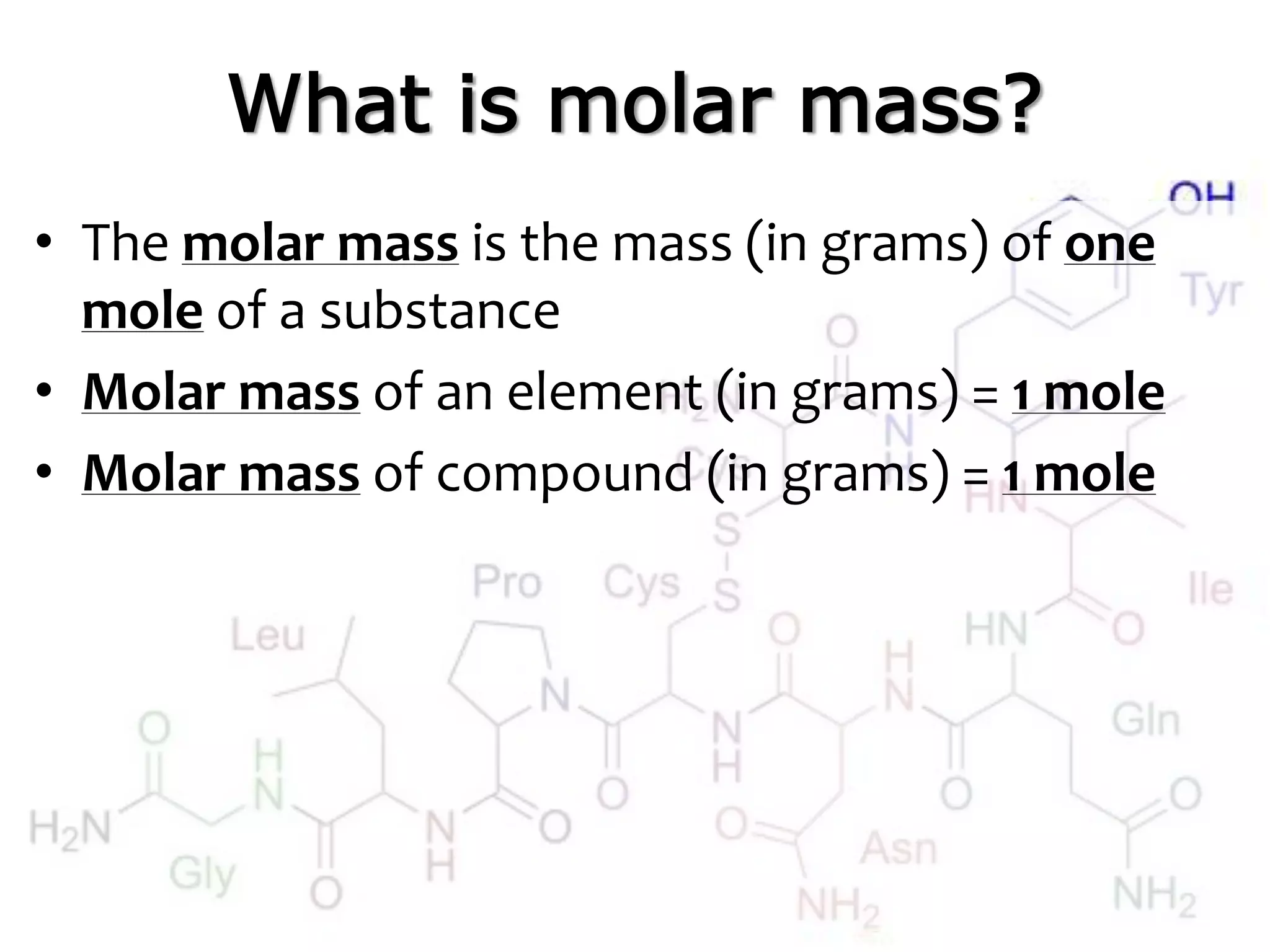
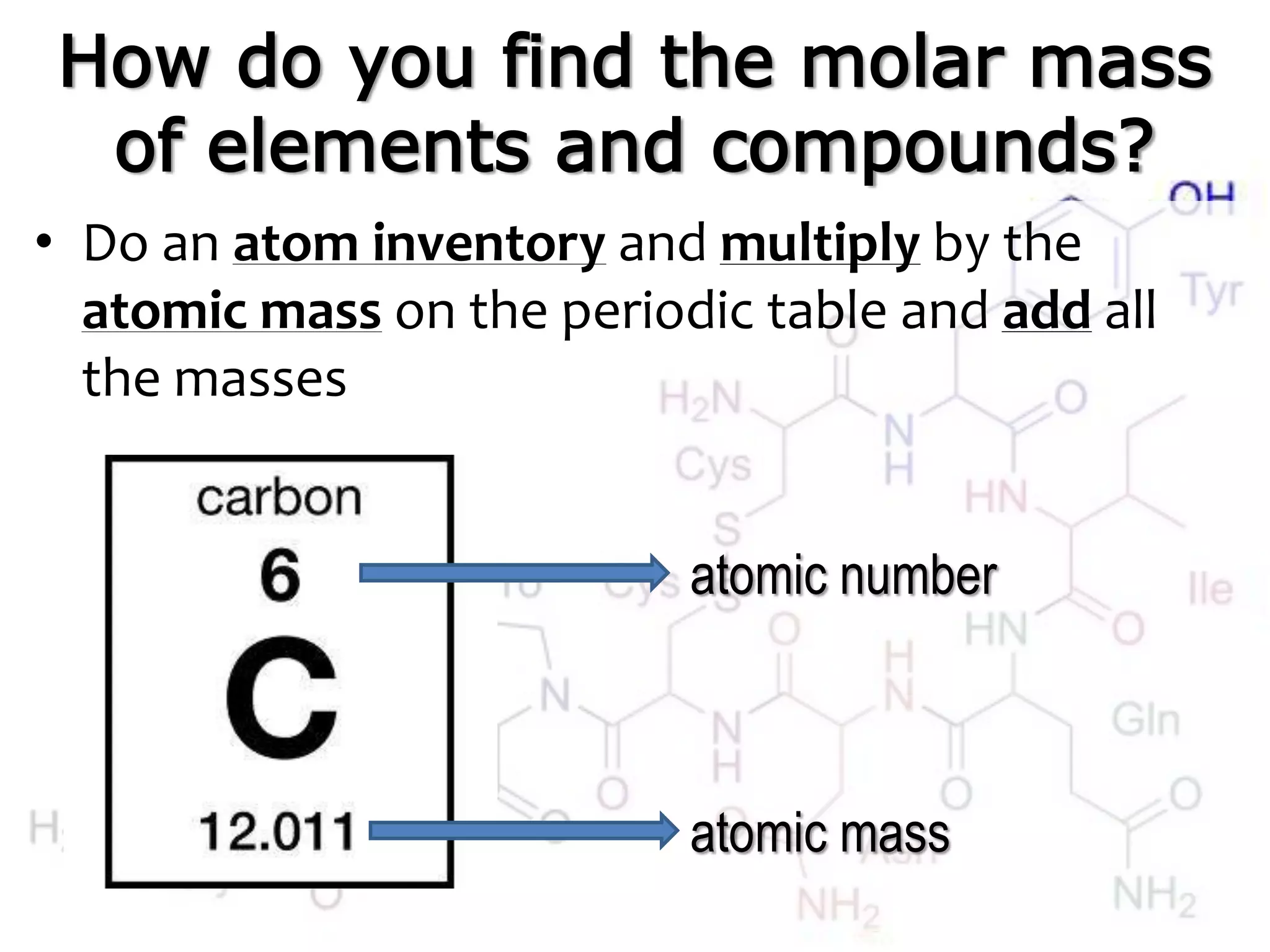
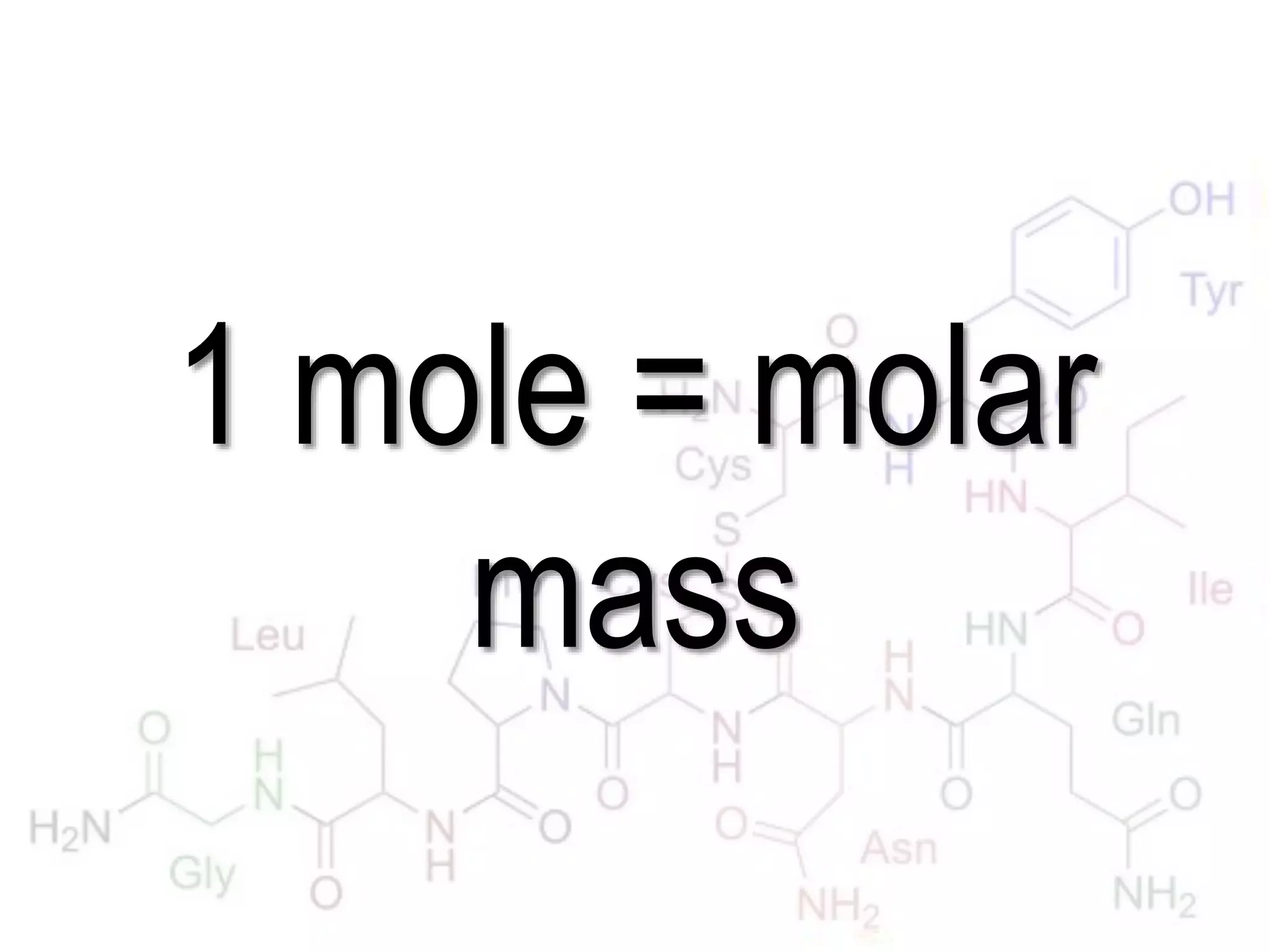
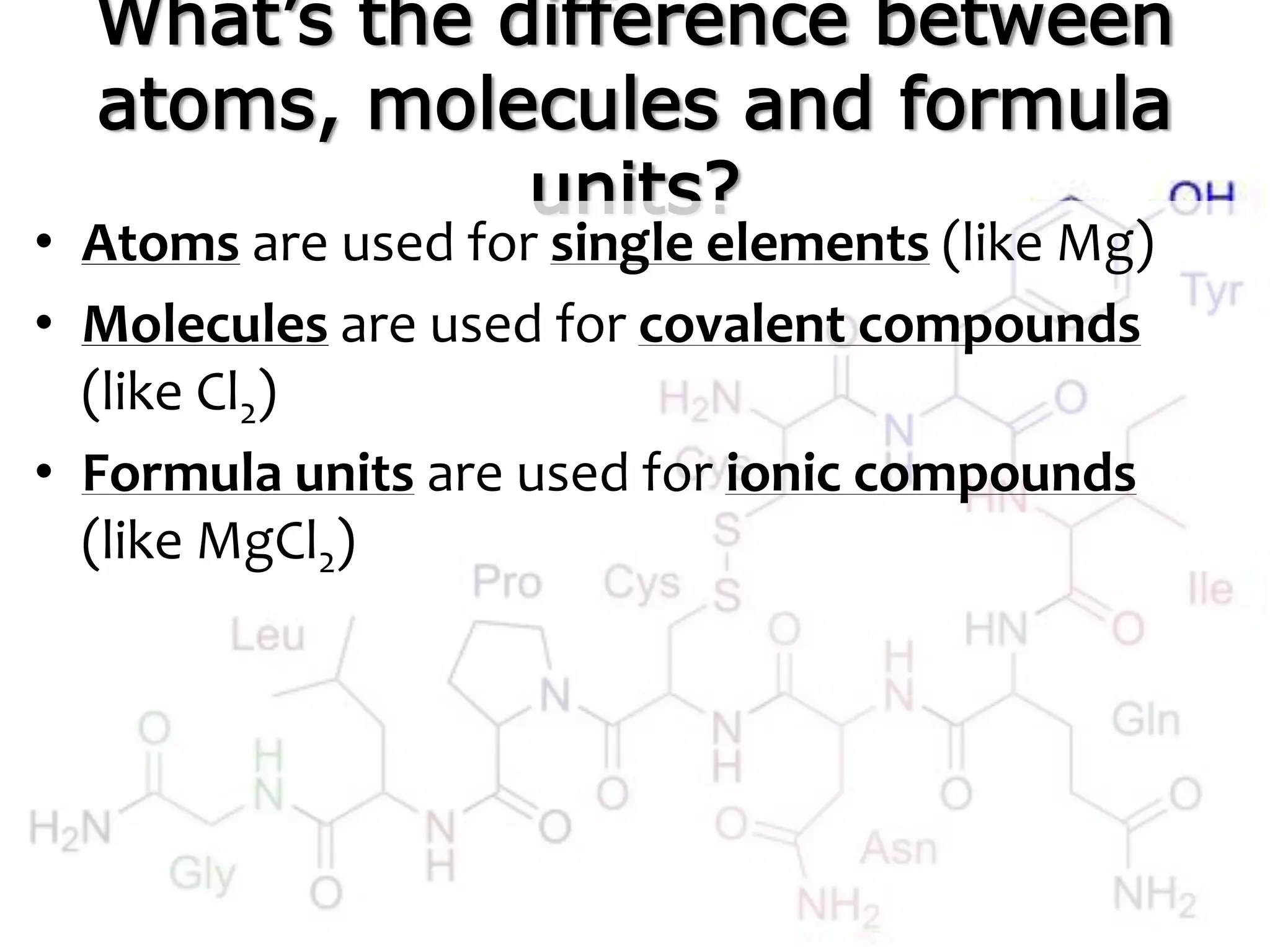
A mole is the standard unit used to measure very large quantities of small particles like atoms and molecules. It represents 6.02 x 1023 particles, which is an immense number that is difficult to comprehend. The molar mass of an element or compound is the mass in grams of one mole of that substance. To find molar mass, one does an atom inventory of the substance and multiplies the number of each type of atom by its atomic mass on the periodic table, then sums the results.