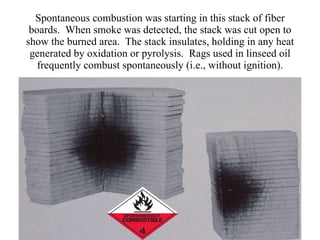
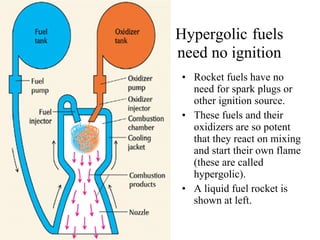
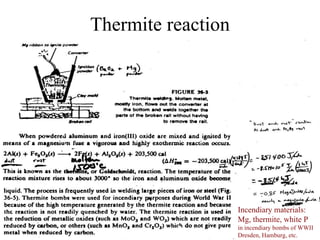
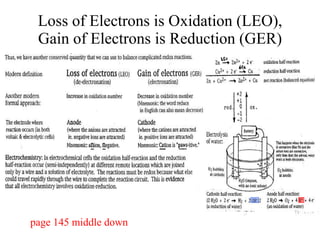
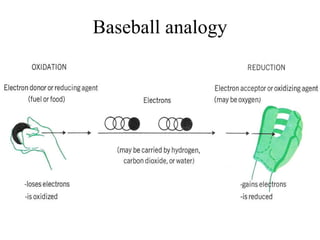
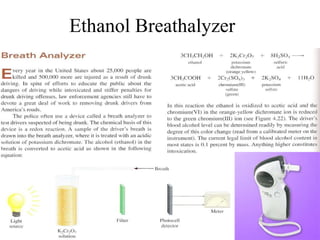
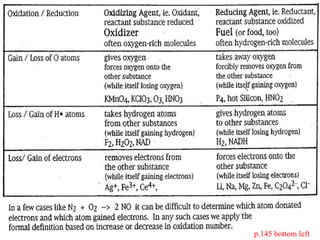
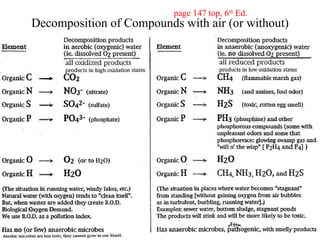
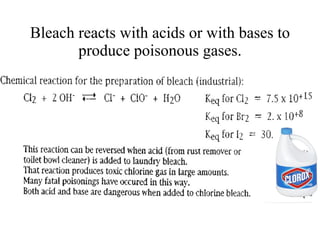
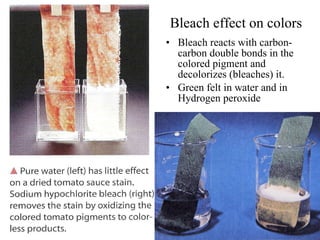
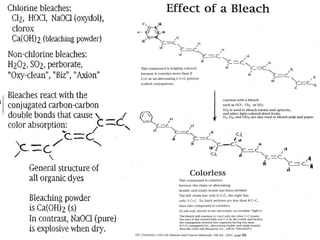
The document discusses oxidation-reduction (redox) reactions, including definitions and examples. It covers topics like rusting, fire, combustion, fuels, ignition sources, and both everyday and hazardous chemical reactions involving oxidation. Color changes and the role of elements like oxygen, chlorine, and metals are also examined in the context of redox processes.
























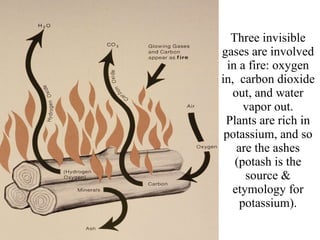

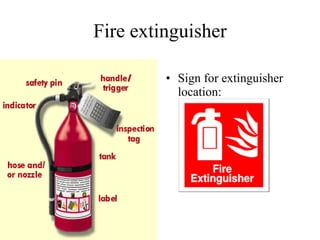
![To extinguish a fire, one must remove one (or more) of the three requirements Remove the fuel (spread it out, cover it). Remove the oxygen (cover the fire with a solid lid, smother it with carbon dioxide or water). Remove the heat (soak it with water, spread it out) Remove free radicals (radicals are needed for flames) [4th requirement would use a flame tetrahedron]](https://image.slidesharecdn.com/redox-100317222351-phpapp01/85/Redox-39-320.jpg)