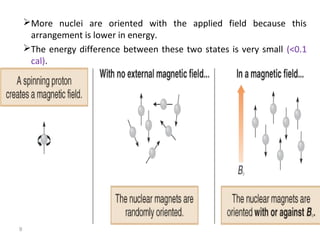
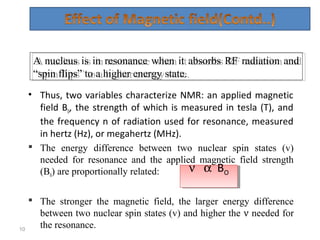
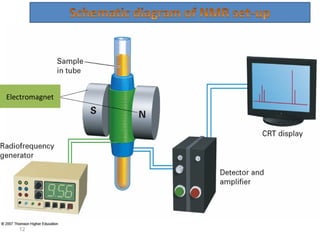
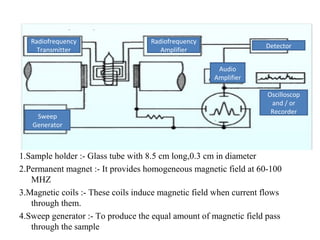
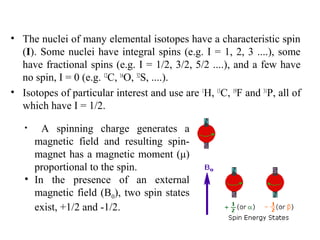
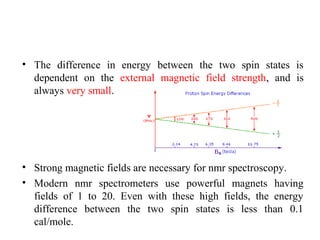
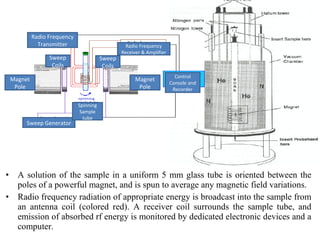
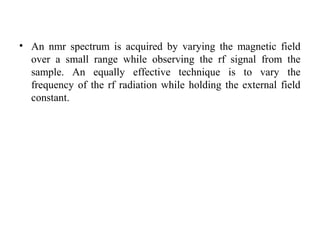
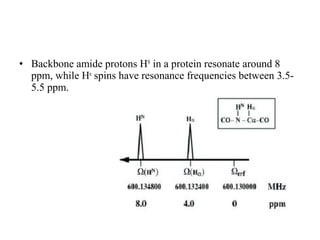
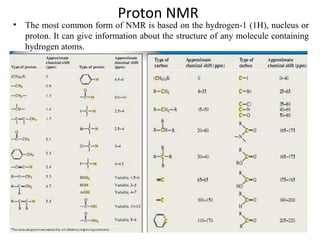
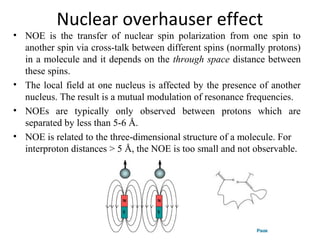
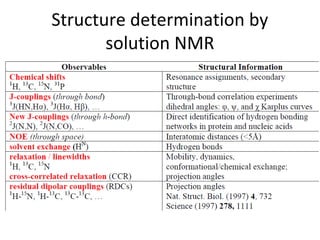
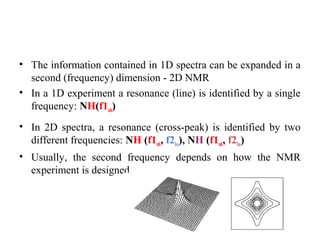
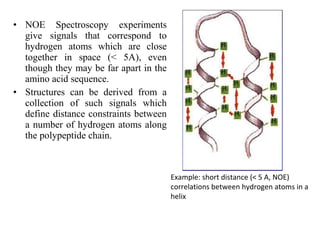
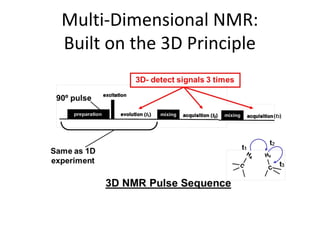
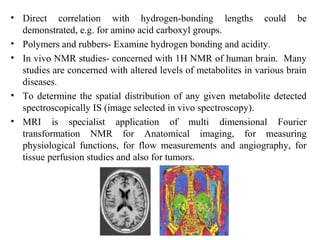
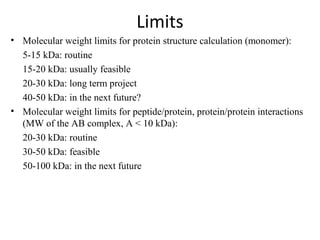
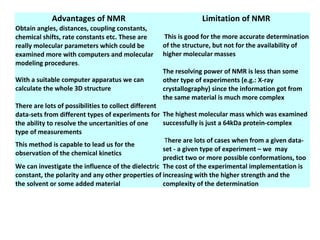
Nuclear magnetic resonance (NMR) spectroscopy exploits the magnetic properties of atomic nuclei. It can provide information about the physical and chemical properties, structure, dynamics, and kinetics of biochemical systems. NMR spectroscopy works by aligning atomic nuclei with an external magnetic field and then stimulating them with radiofrequency pulses. This causes the nuclei to absorb and emit radiofrequency radiation at characteristic frequencies. Analyzing these frequencies yields information about the molecule's structure. NMR is widely used to determine the structures of organic molecules and biomolecules like proteins and nucleic acids.