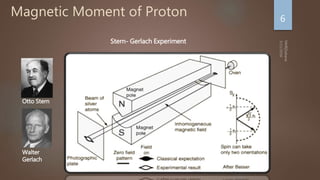
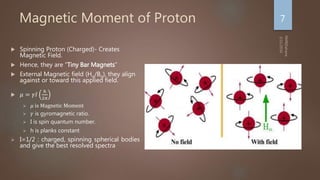
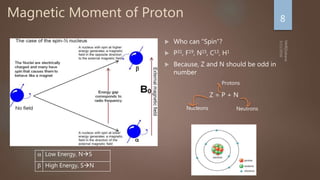
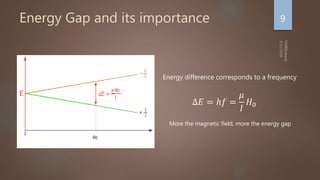
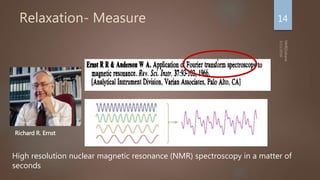
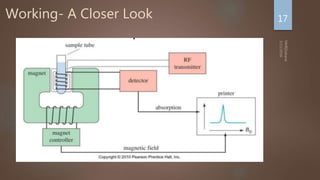
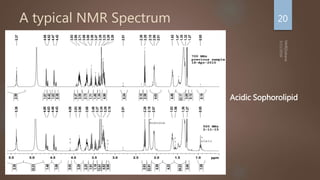
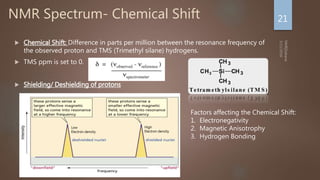
Nuclear magnetic resonance (NMR) spectroscopy uses magnetic fields and radiofrequency pulses to analyze atomic nuclei and determine molecular structures. NMR works by aligning atomic nuclei in an external magnetic field and measuring their signals as they relax back to equilibrium. The signals provide information on chemical shifts, spin-spin couplings, and molecular relaxation times that can be used to elucidate molecular structures. Modern NMR techniques including Fourier transforms, multidimensional experiments, and magnetic resonance imaging (MRI) have significantly advanced structural analysis and medical applications of NMR spectroscopy.



![Why NMR? [Applications]
• Identification
• Analysis of
material
• Protein
Structure
• Kinetics
• Condensed
Matter Physics
• MRI
Medical Physics
ChemistryBiology
5](https://image.slidesharecdn.com/0e34f36d-29af-45f2-a427-882ccdba6807-160311051735/85/Introduction-to-NMR-5-320.jpg)





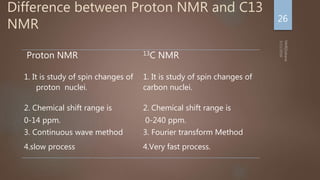
![NMR Spectrum- Interpretation
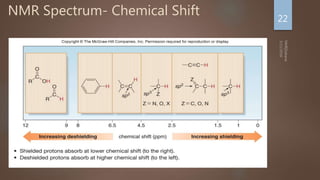
Number of signals: Indicates how many different kinds of
protons are present
Position of signals: Indicates Magnetic environment of the
signal
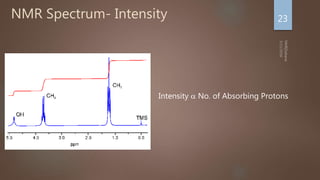
Relative Intensity: Proportional to number of protons present
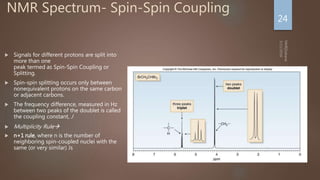
Splitting: Indicates number of splitting Nuclei [Usually
Protons]
25](https://image.slidesharecdn.com/0e34f36d-29af-45f2-a427-882ccdba6807-160311051735/85/Introduction-to-NMR-25-320.jpg)