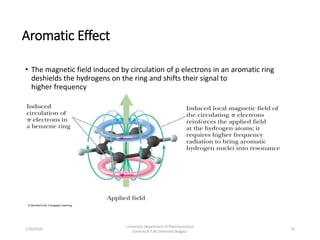
This document provides an overview of NMR spectroscopy. It discusses the basic principles of NMR, including nuclear spin and how nuclei align in magnetic fields. It also describes NMR instrumentation and how NMR spectra provide information about a molecule's structure. Specifically, it discusses chemical shifts and how they are influenced by functional groups. Additionally, it covers topics like spin-spin coupling, 2D NMR techniques including COSY and HETCOR, and how NMR can be used to determine molecular structure.