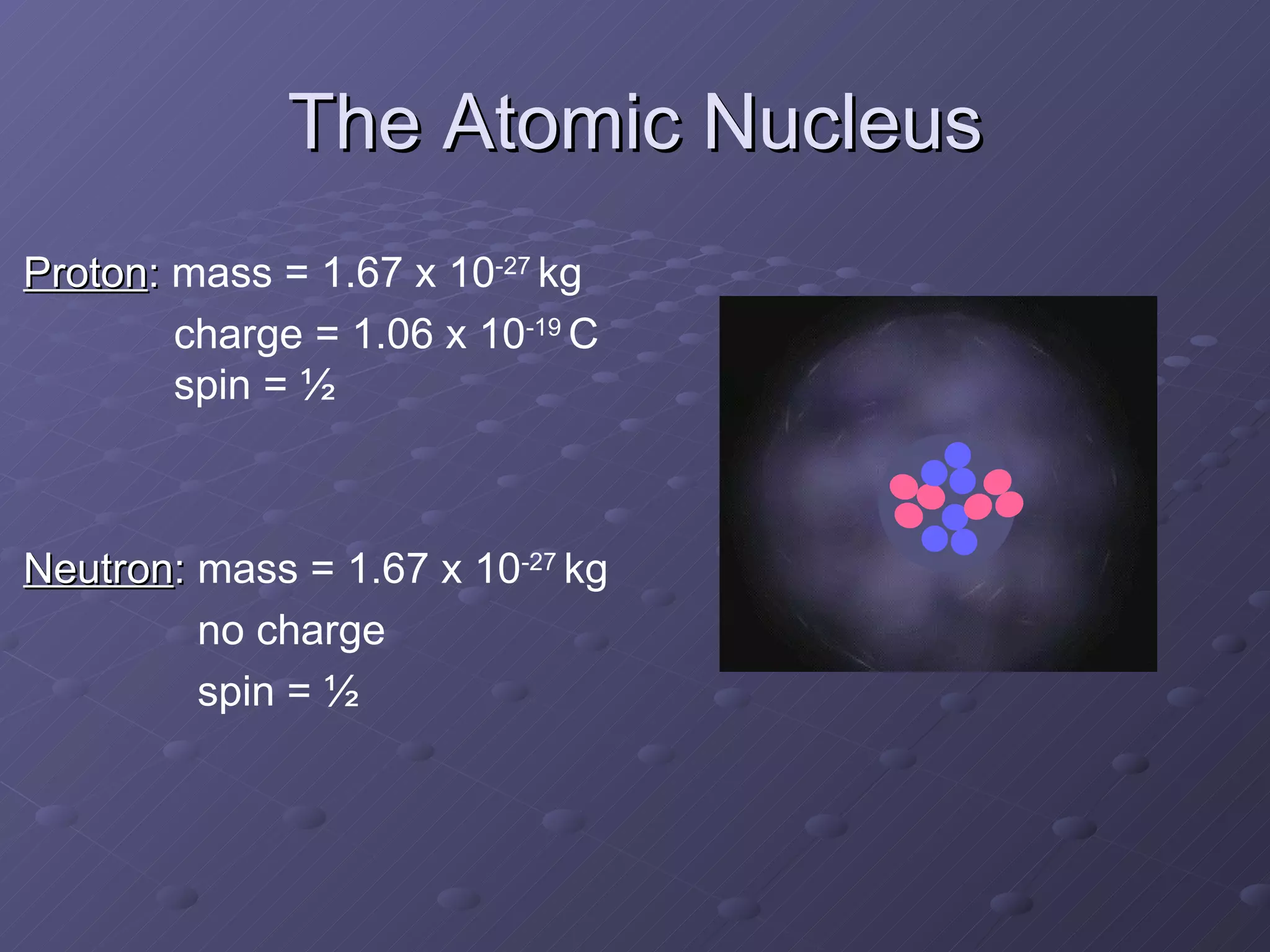
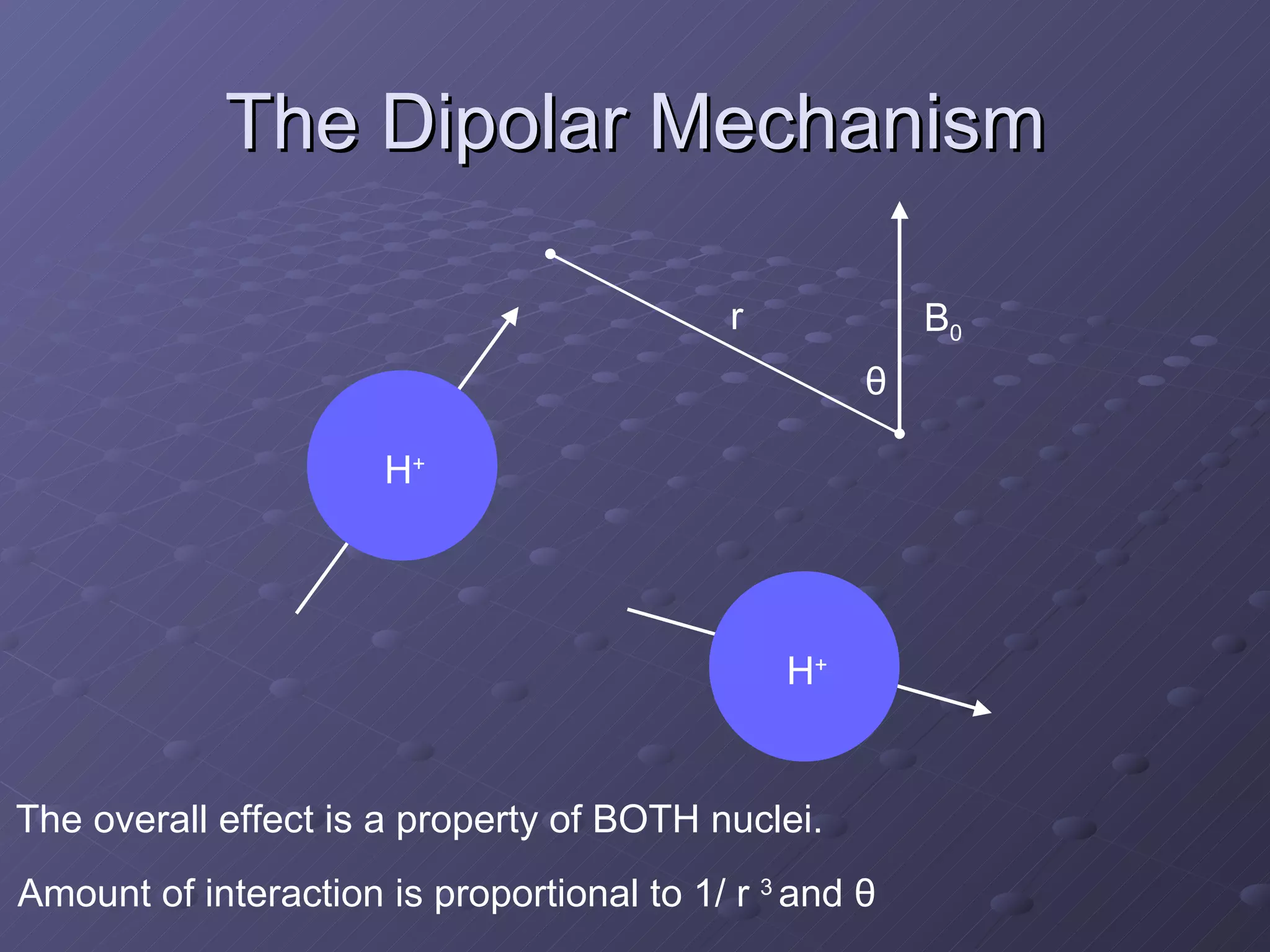
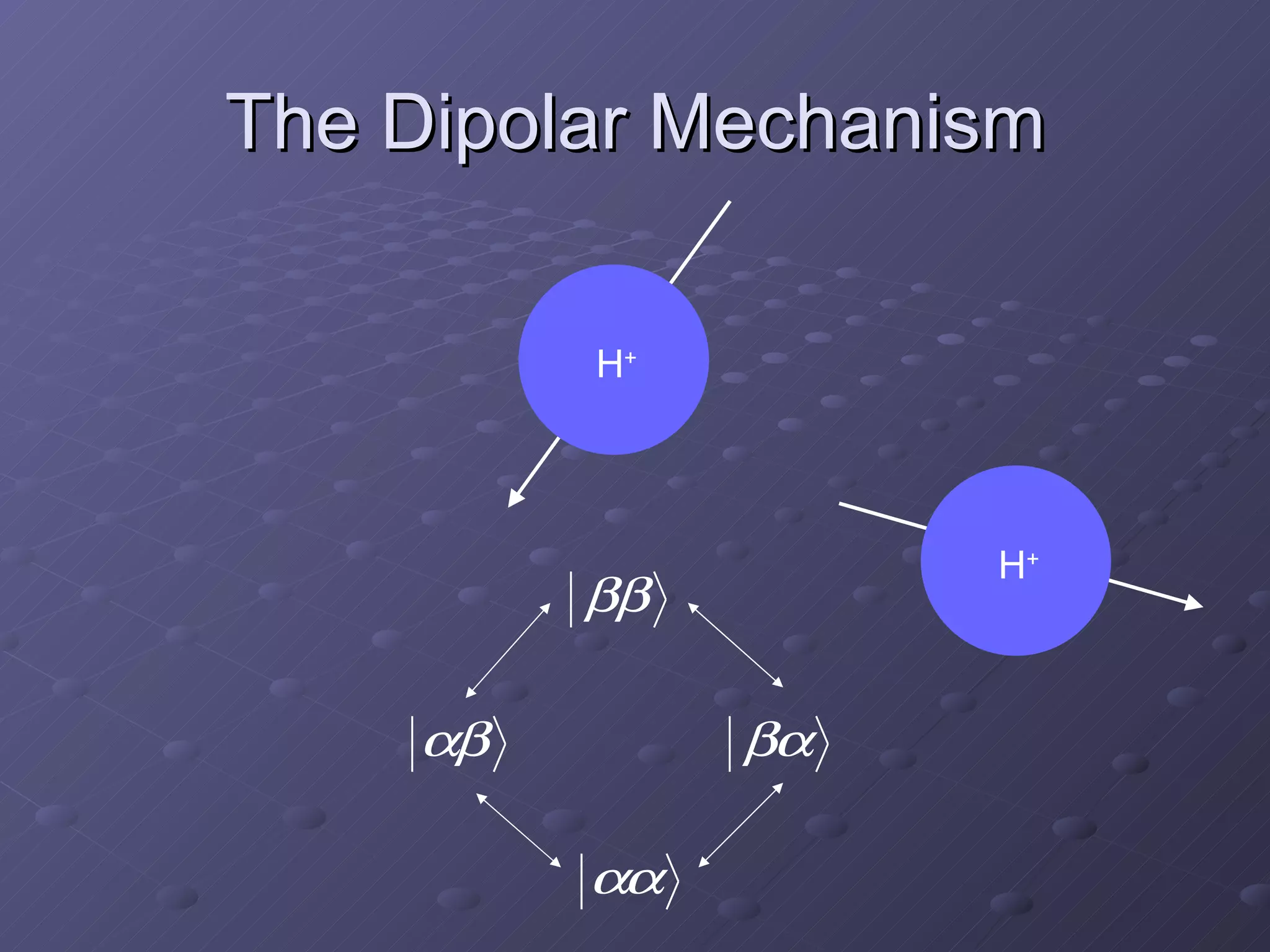
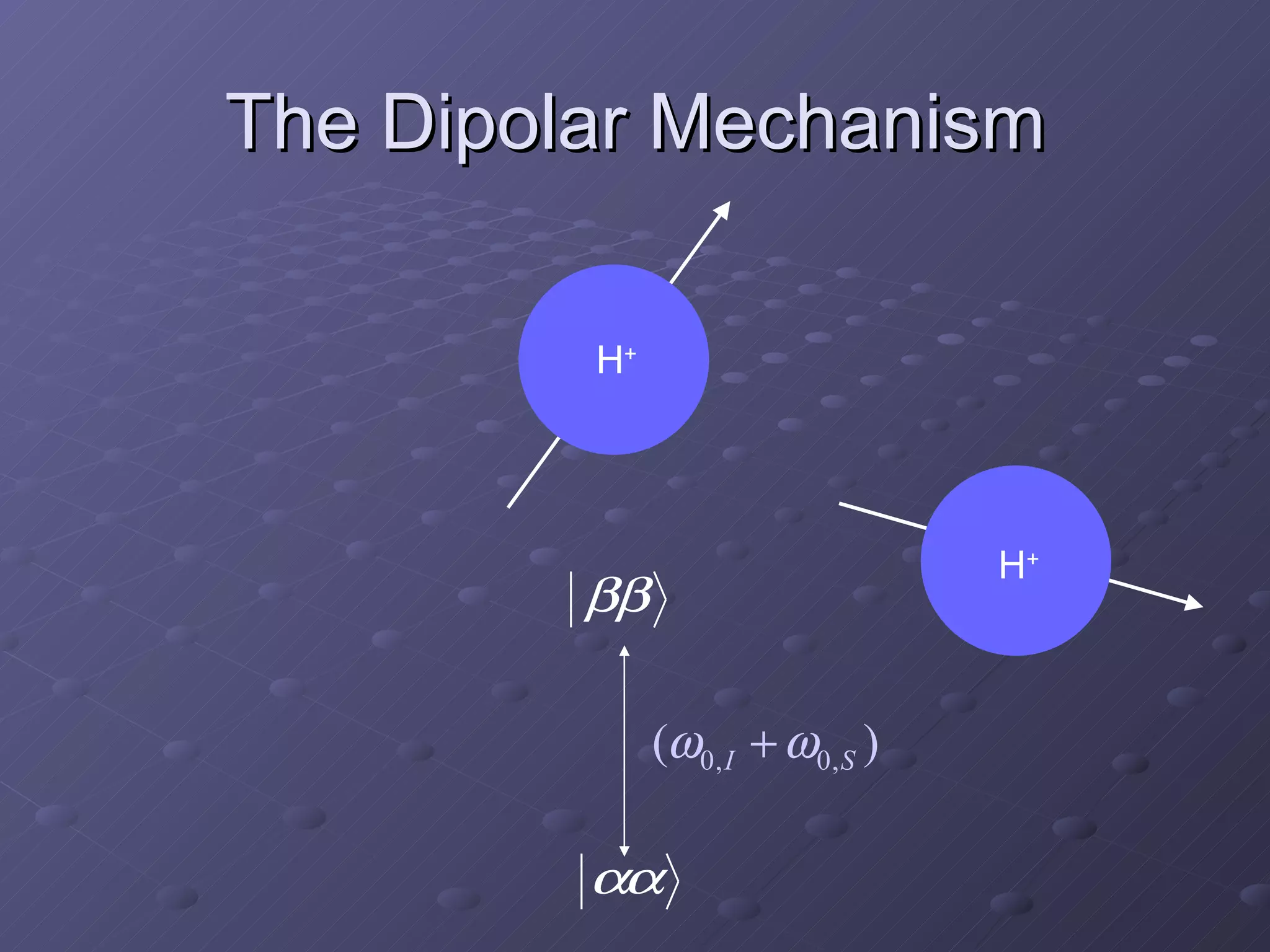
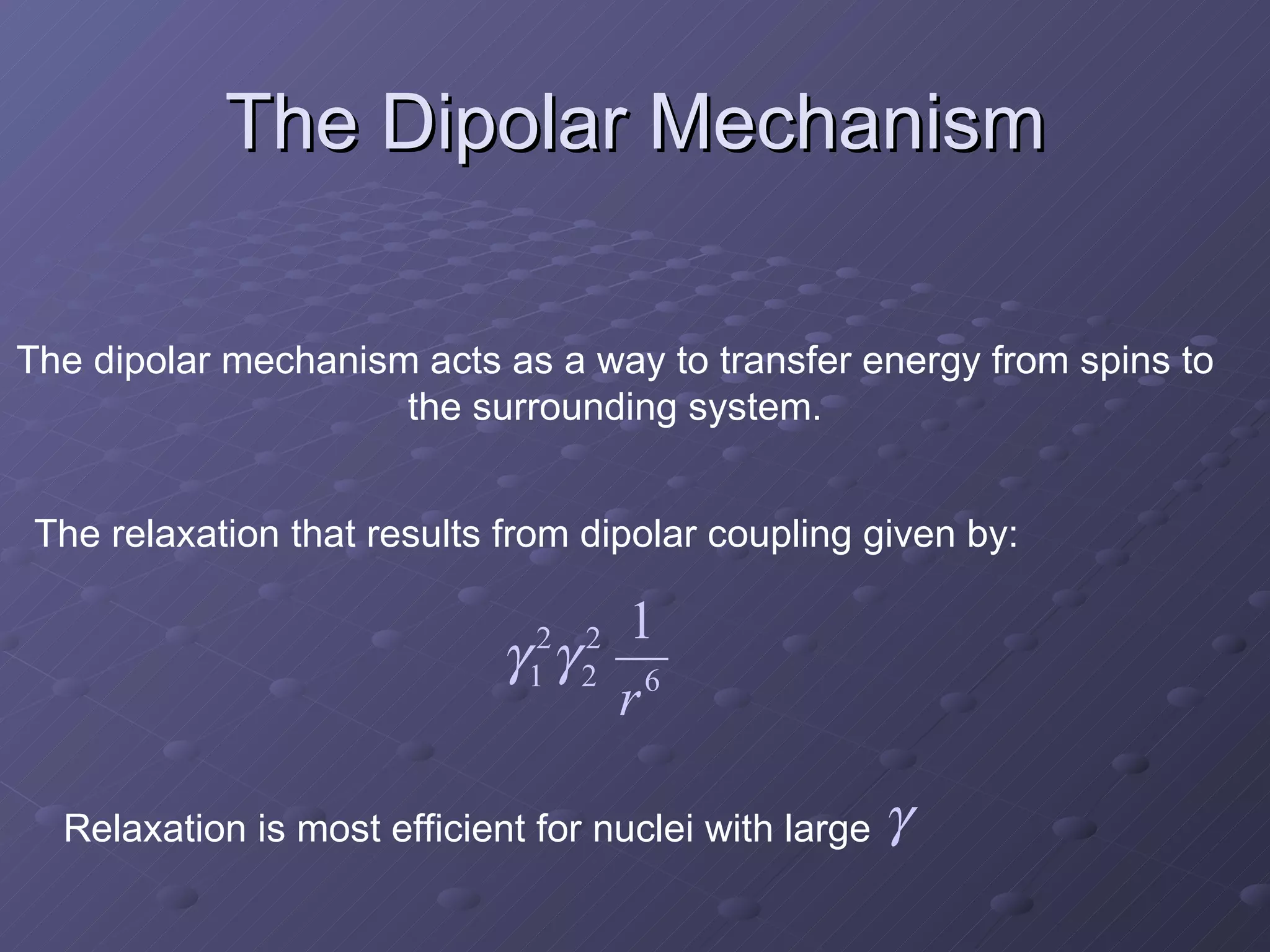
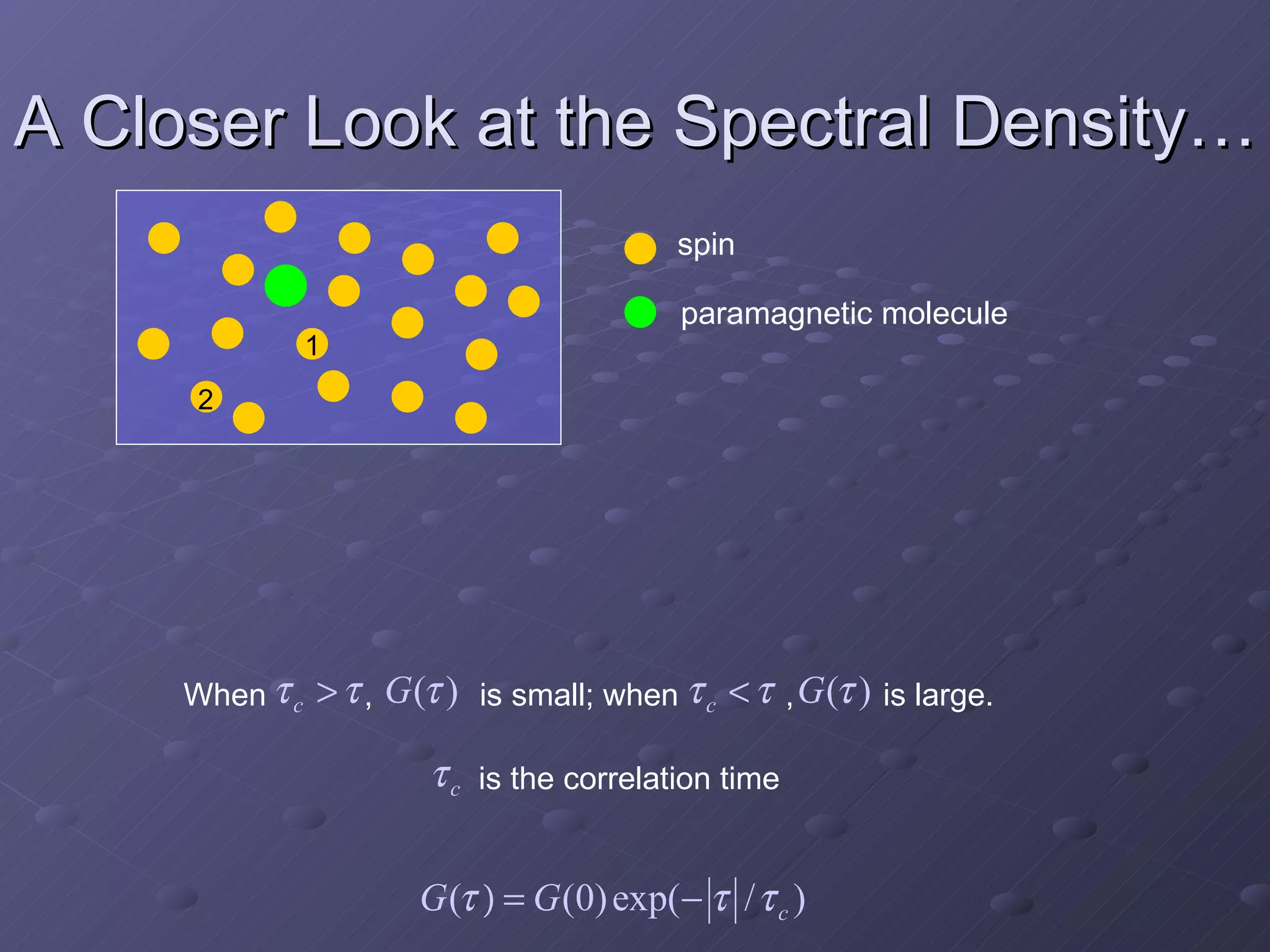
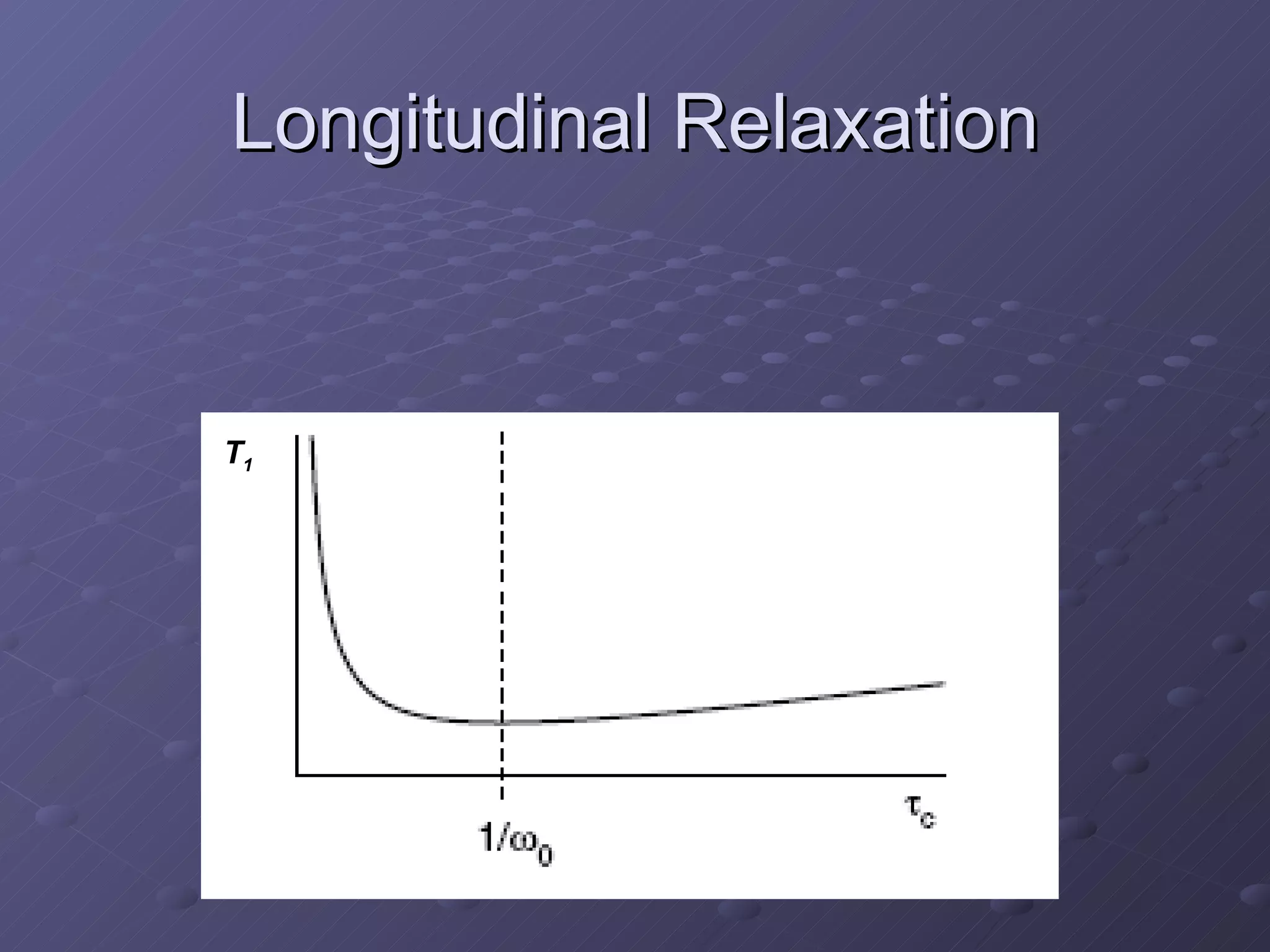
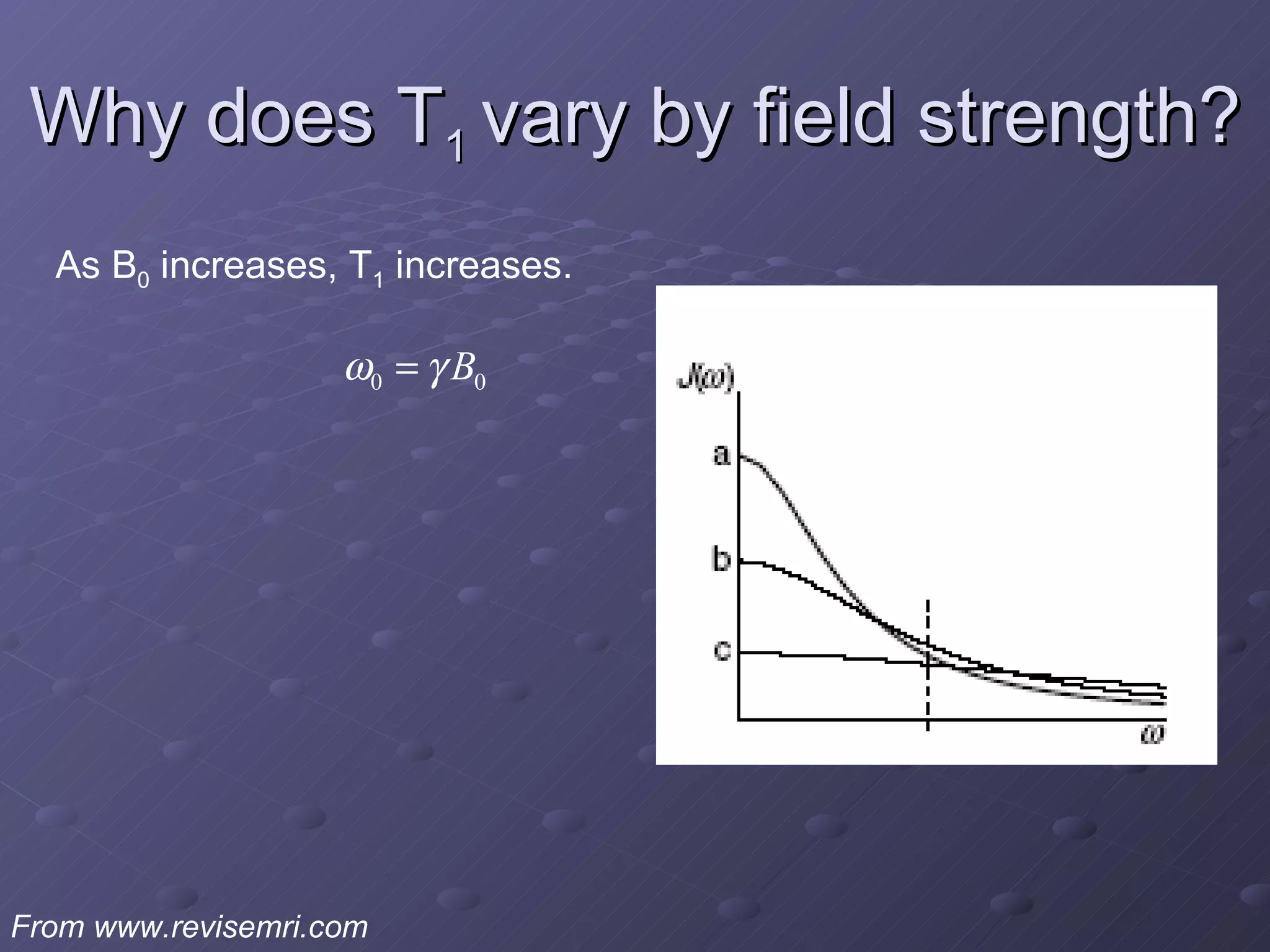
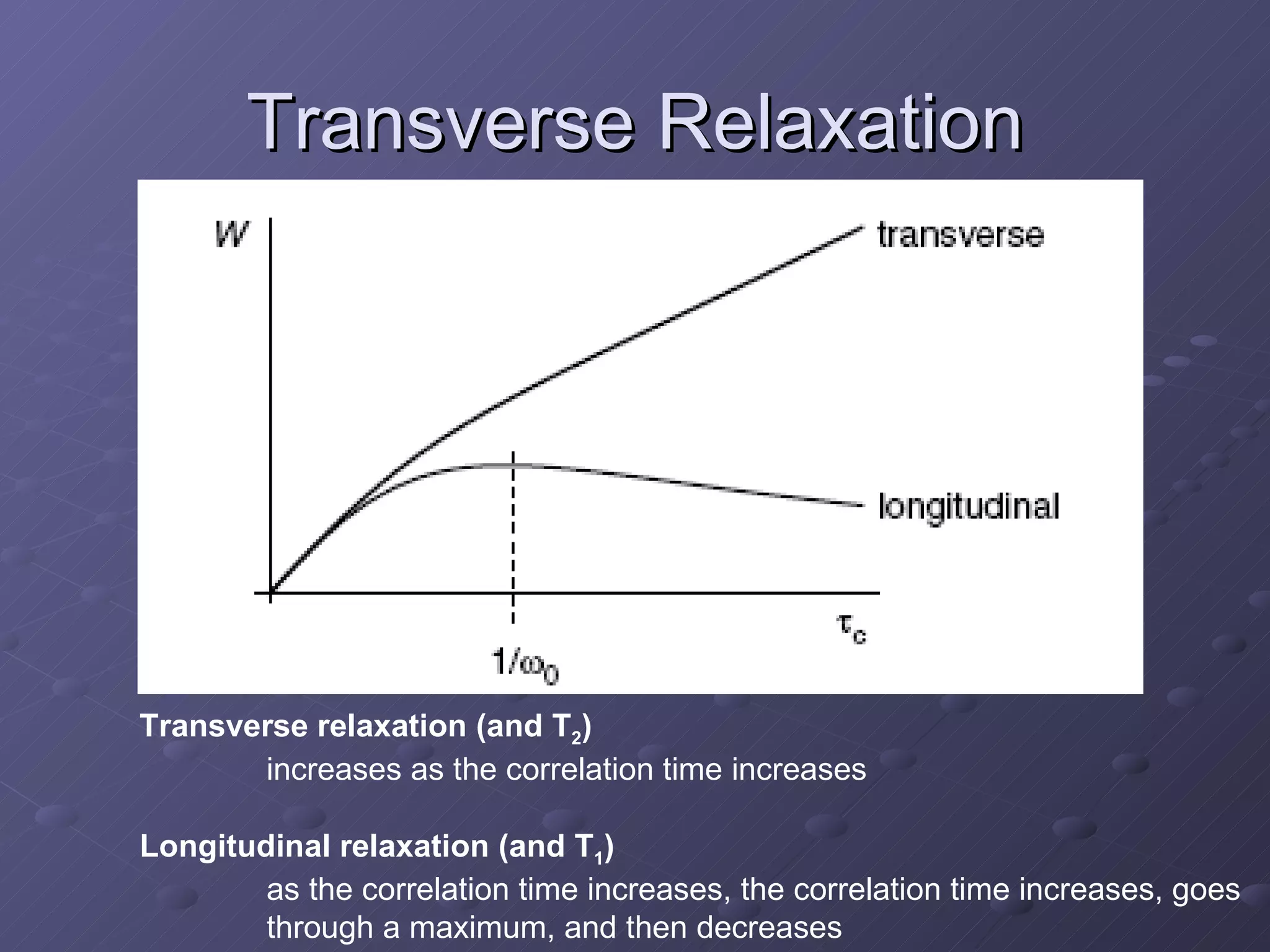
This presentation discusses nuclear spin and relaxation in NMR, detailing the structure and properties of protons and neutrons. It explains longitudinal and transverse relaxation processes, their causes, and factors affecting T1 and T2 relaxation times in different tissues. The document also touches on quantum mechanics and the role of spectral density in relaxation phenomena.