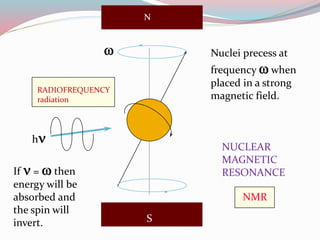
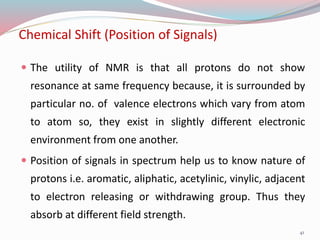
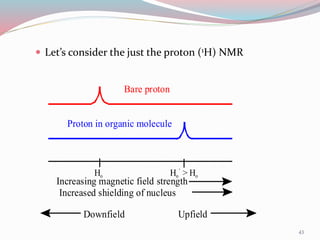
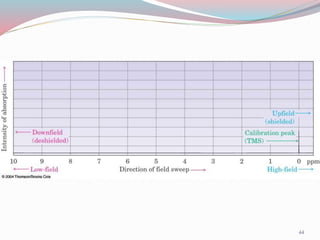
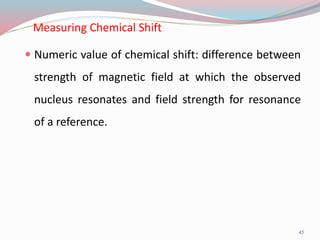
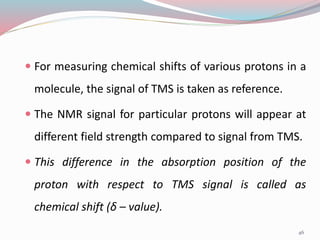
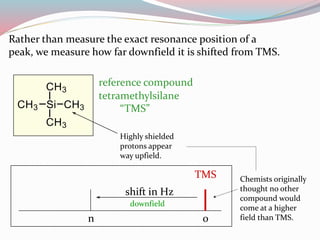
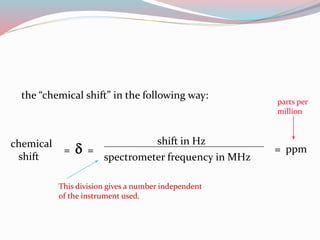
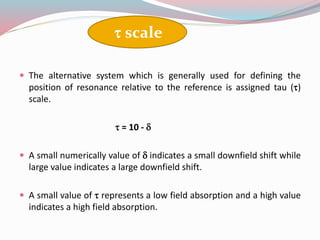
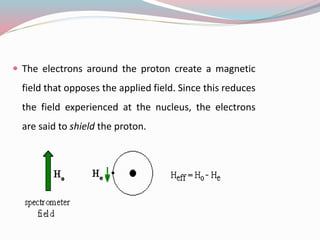
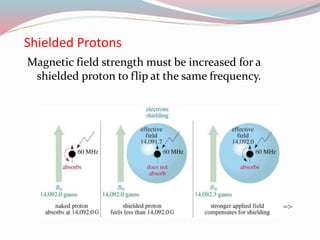
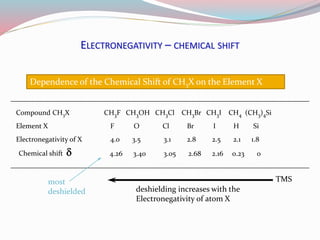
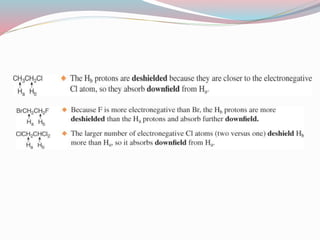
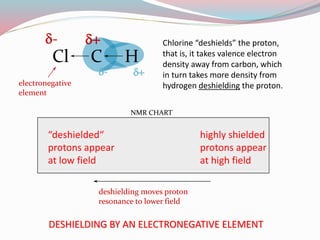
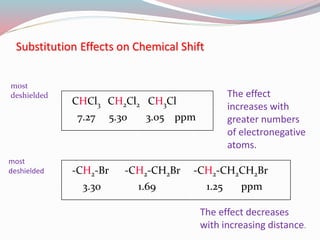
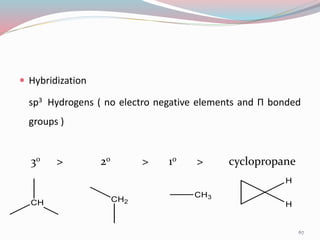
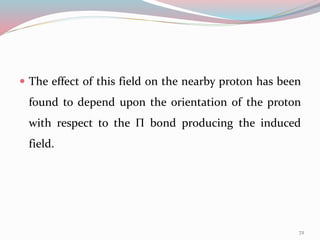
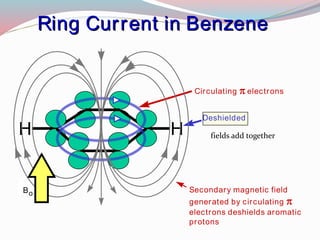
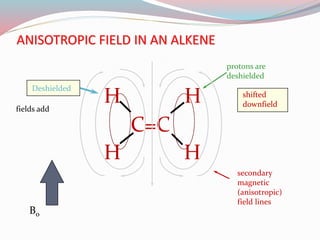
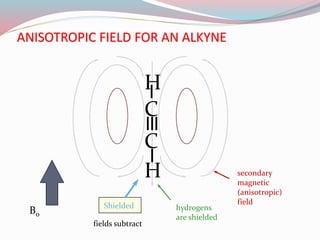
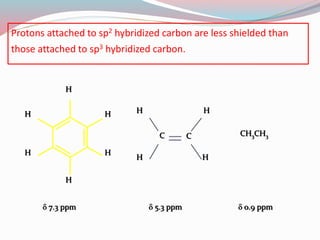
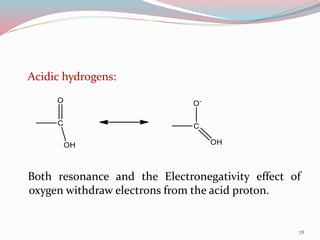
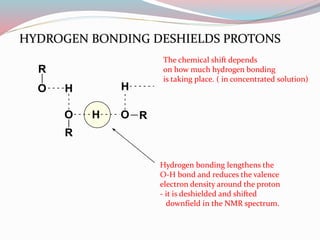
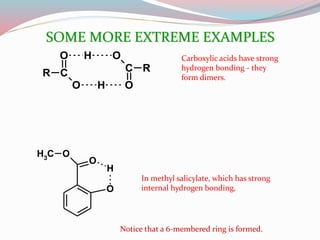
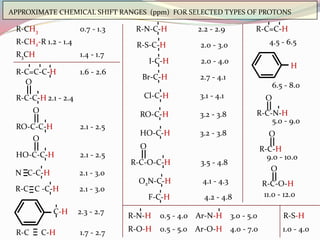
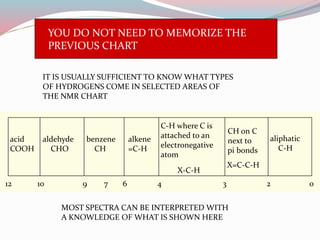
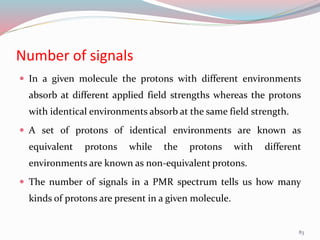
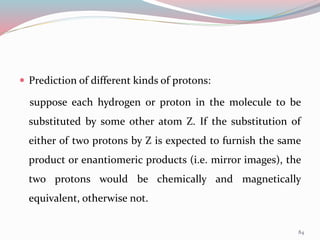
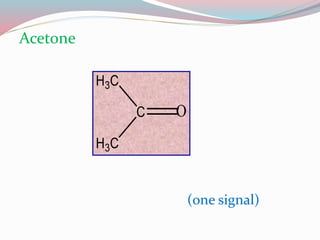
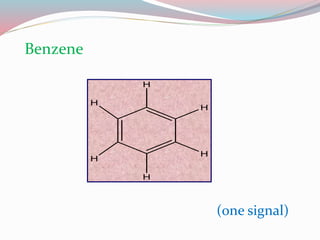
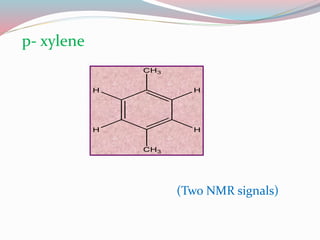
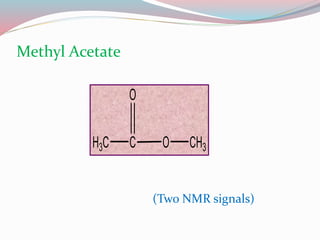
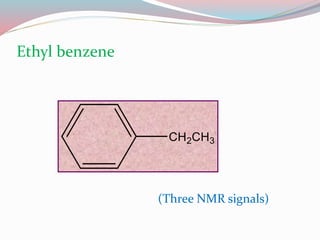
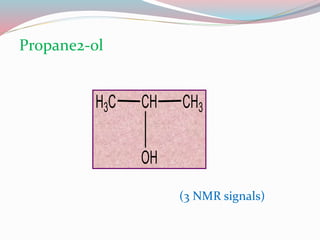
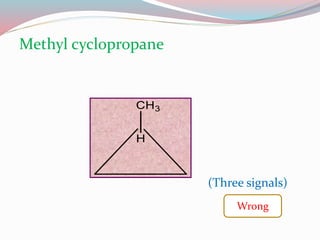
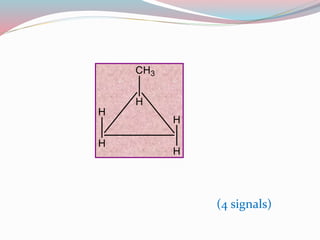
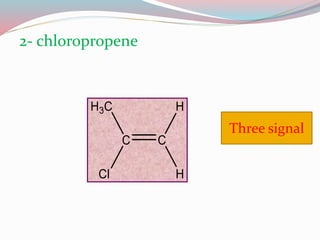
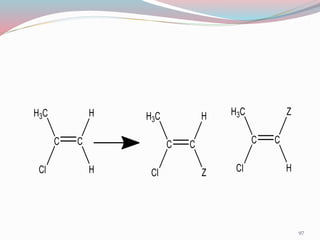
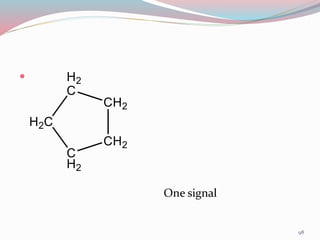
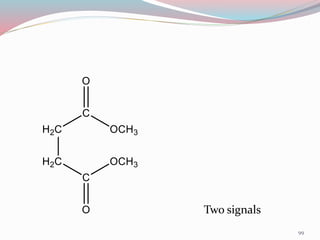
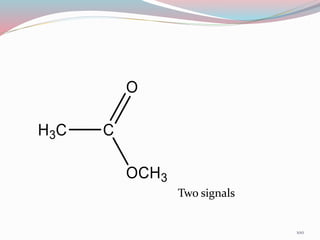
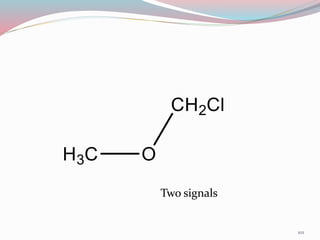
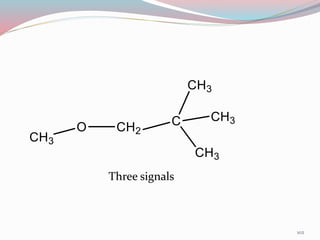
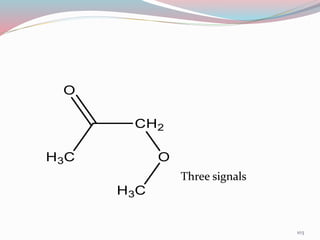
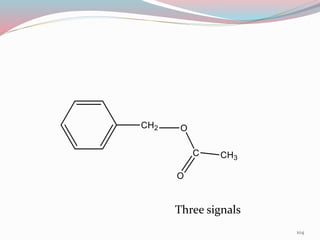
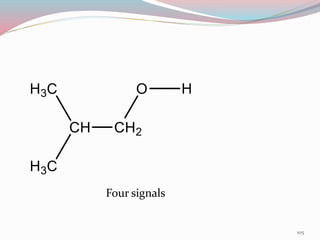
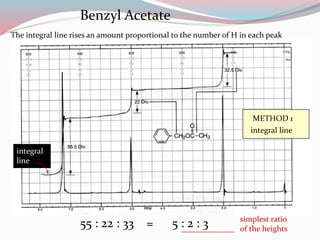
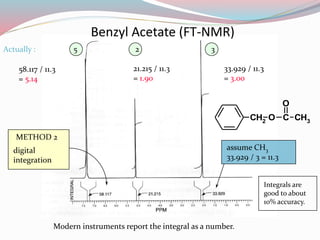
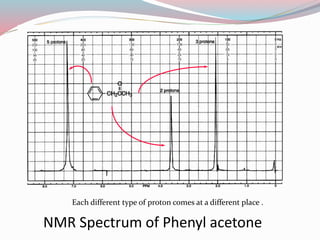
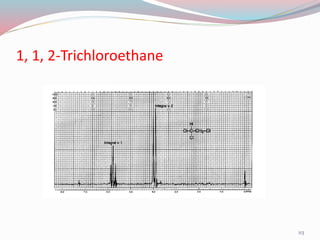
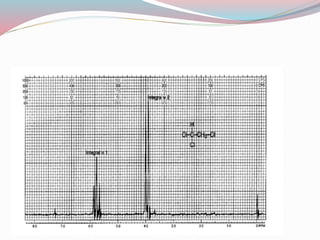
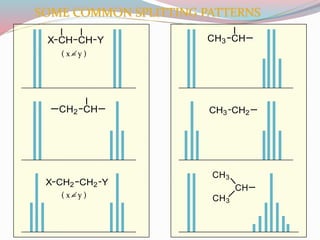
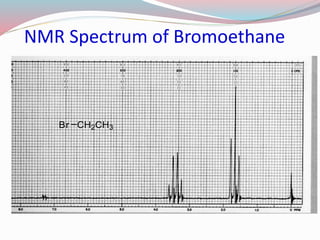
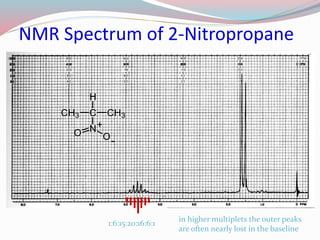
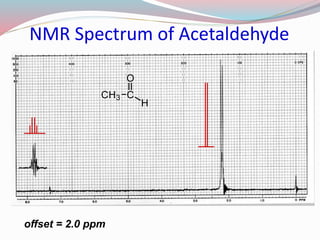
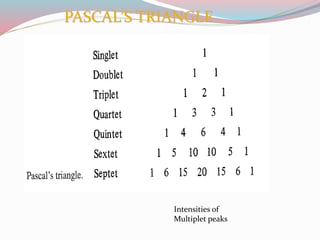
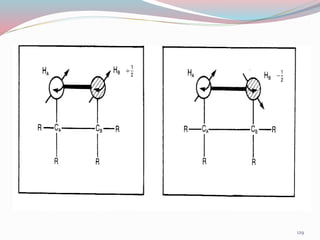
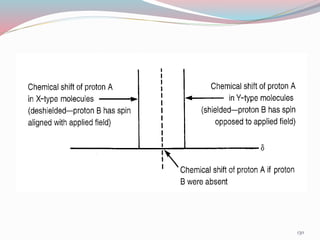
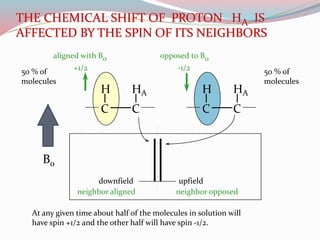
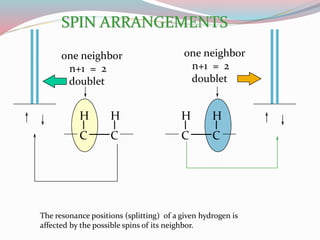
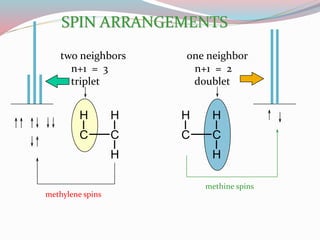
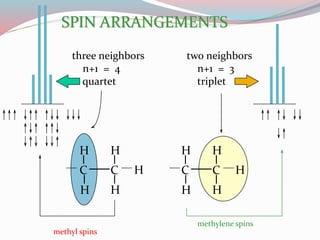
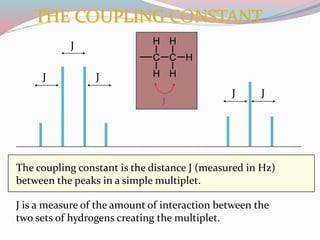
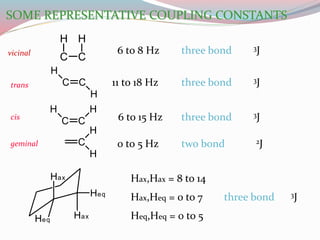
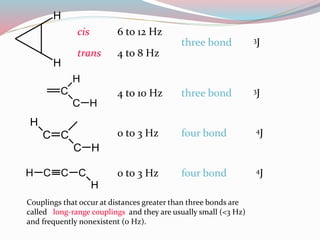
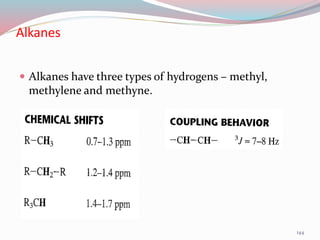
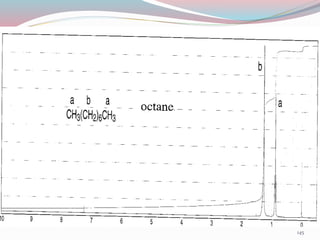
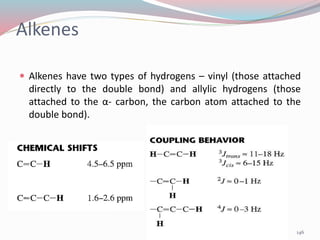
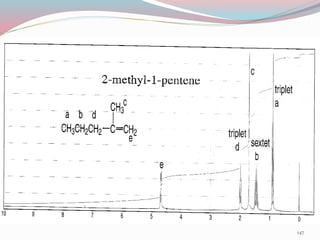
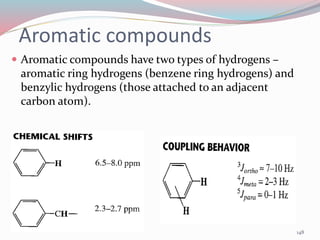
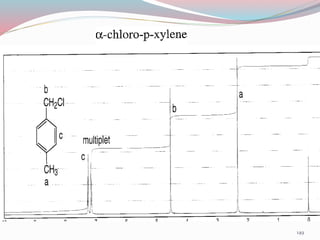
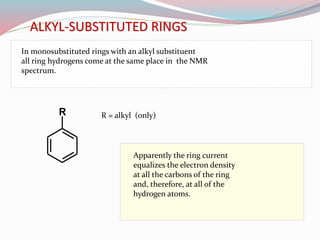
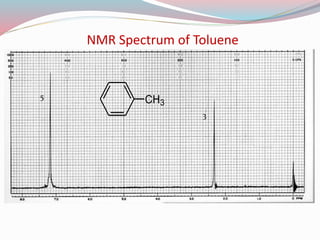
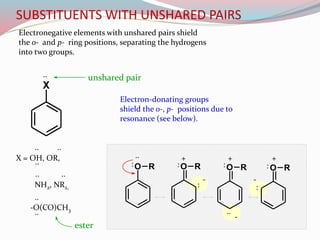
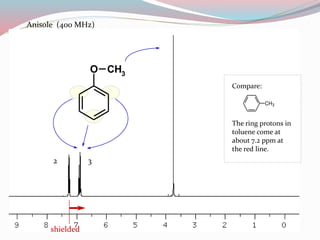
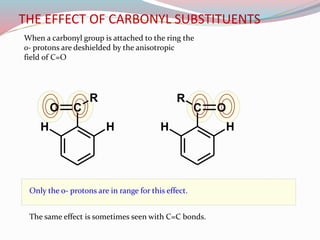
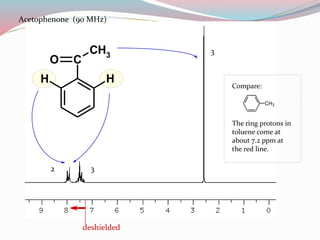
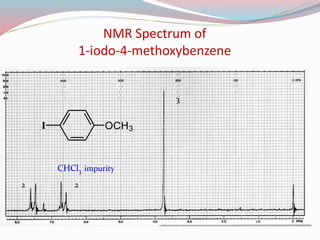
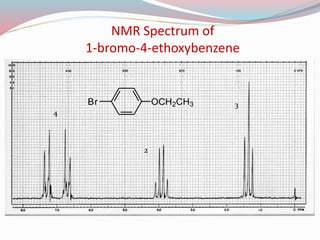
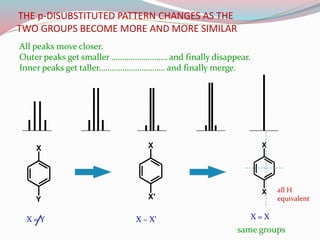
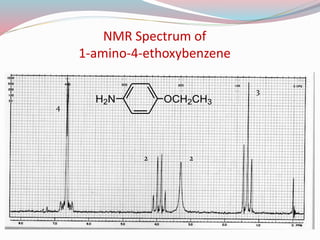
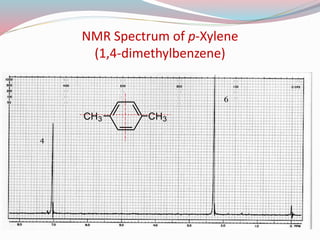
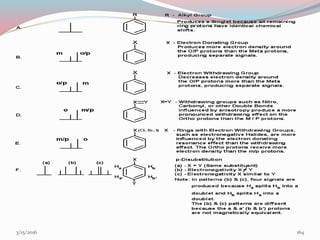
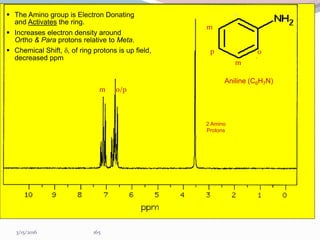
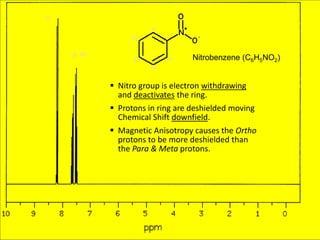
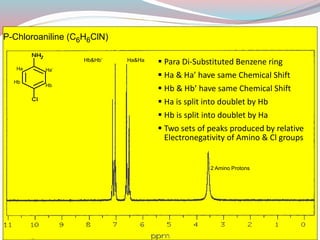
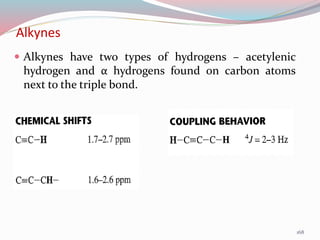
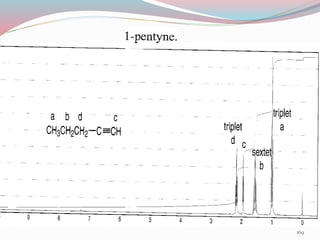
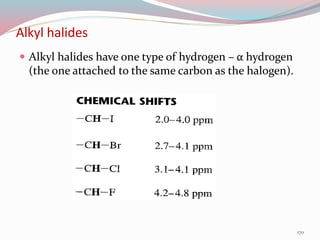
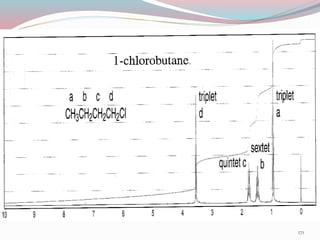
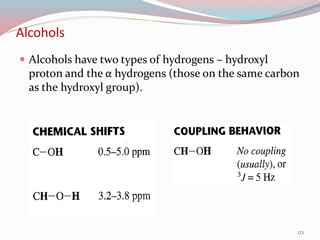
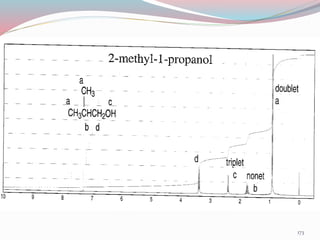
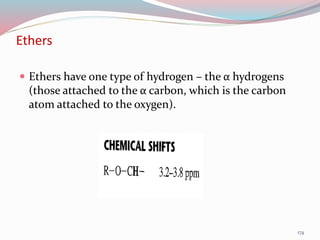
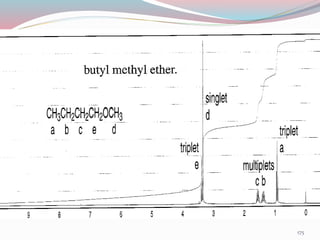
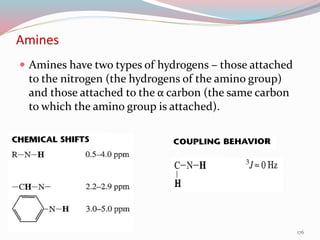
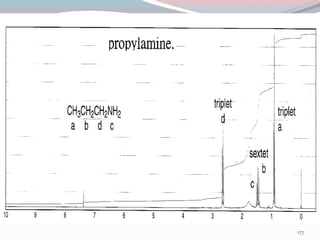
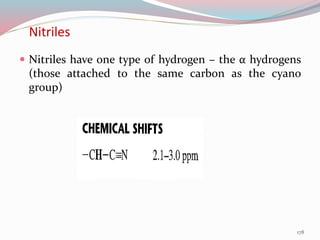
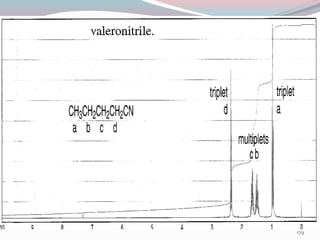
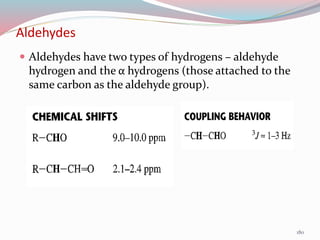
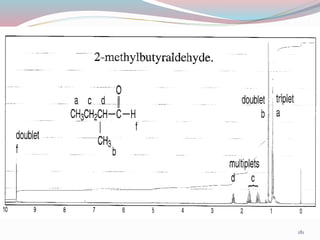
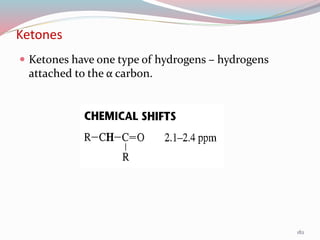
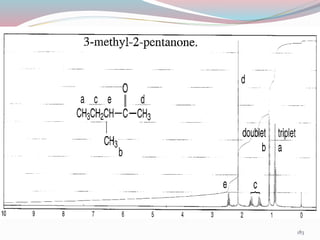
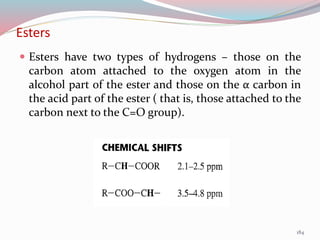
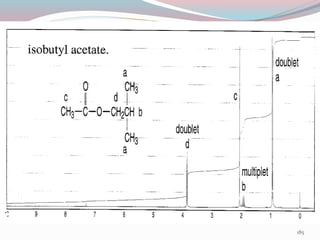
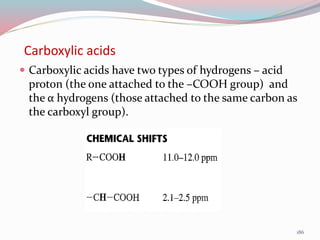
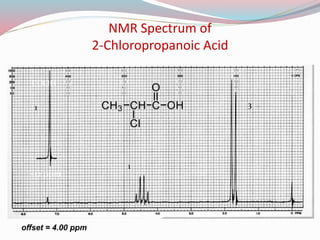
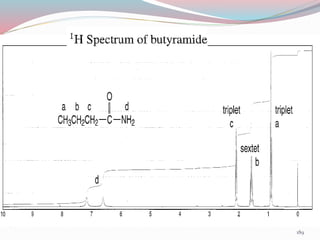
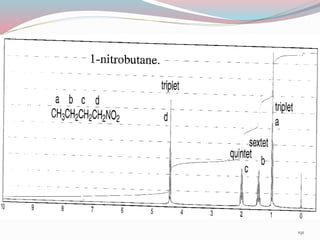
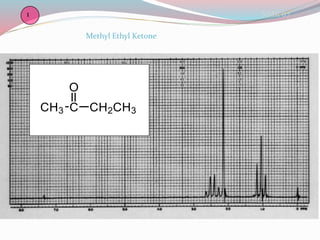
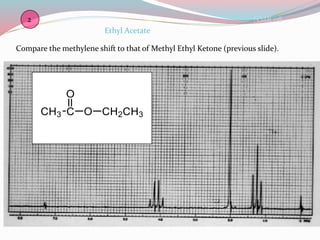
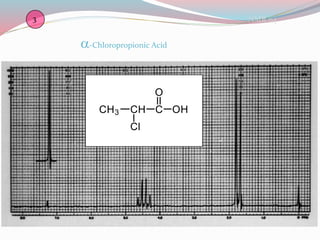
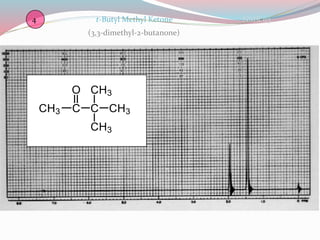
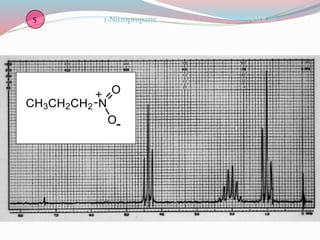
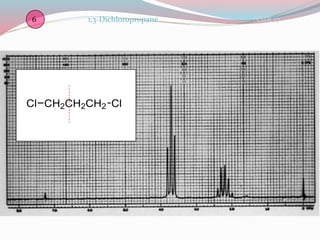
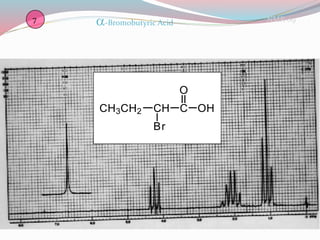
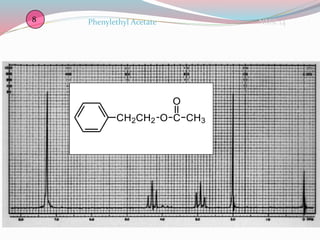
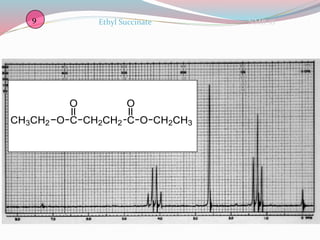
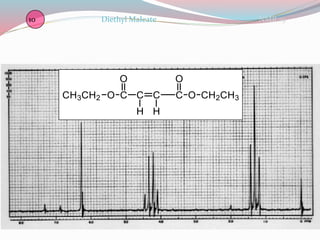
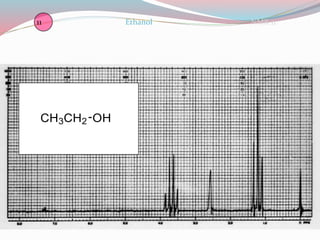
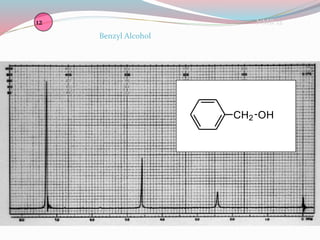
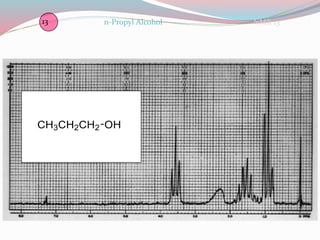
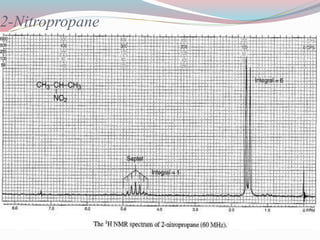
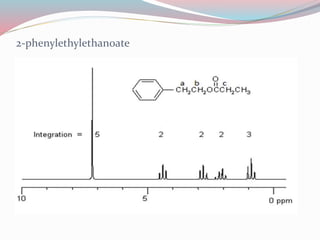
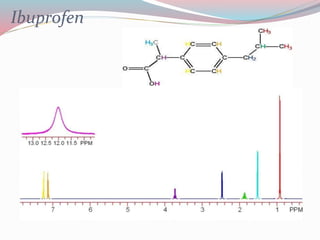
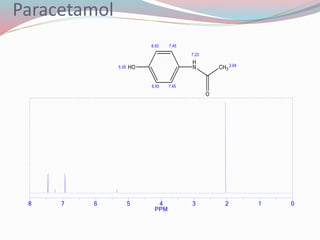
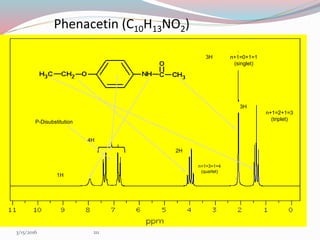
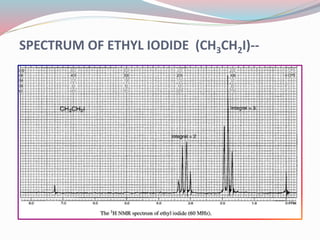
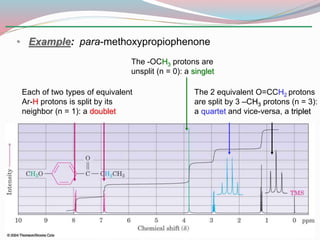
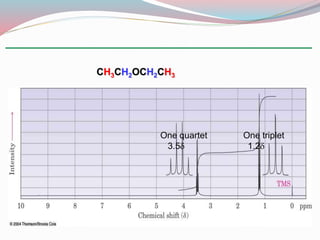
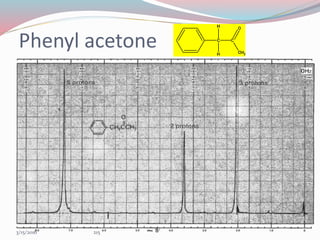
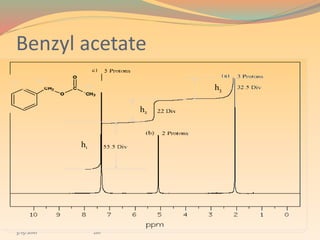
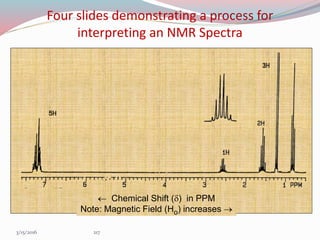
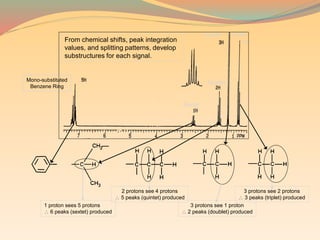
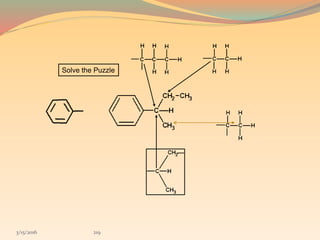
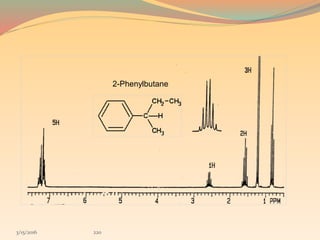
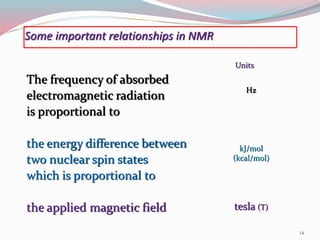
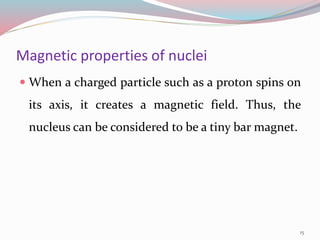
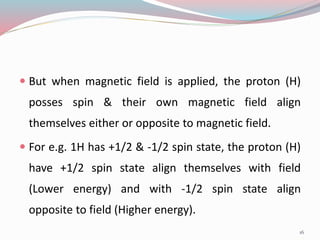
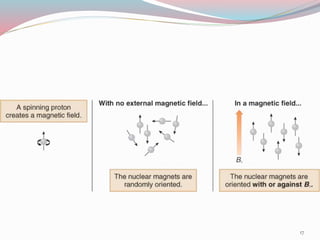
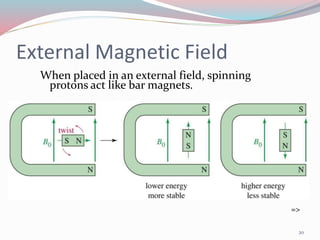
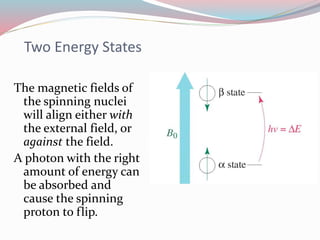
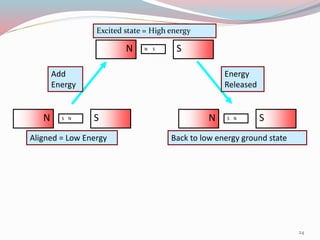
NMR spectroscopy uses radio waves to induce transitions between magnetic energy levels of nuclei in a molecule. When placed in an external magnetic field, nuclei with spin precess at the Larmor frequency, which is proportional to the field strength. Absorption of radio waves at the Larmor frequency causes spin flipping between energy levels. The NMR spectrum provides information on chemical environments and molecular structure from chemical shifts, peak multiplicities, integrals, and coupling constants.




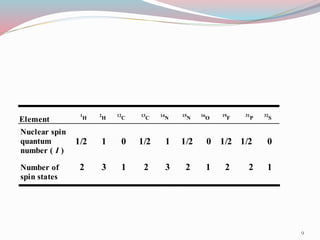
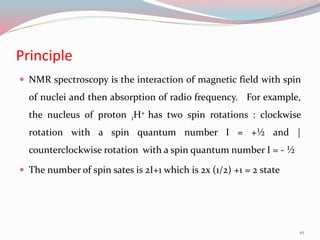

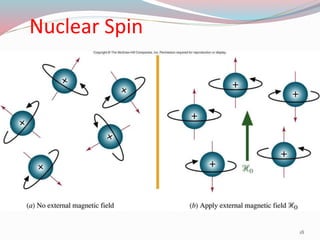

![ According to the quantum theory, a spinning nucleus
can only have values for the spin angular momentum
given by the equation :
Spin angular momentum = [I(I+1)]1/2 h / 2µ
I = Spin quantum number
h= Planck̕s constant
25](https://image.slidesharecdn.com/nmr-160315162509/85/Nuclear-Magnetic-Resonance-Spectroscopy-25-320.jpg)
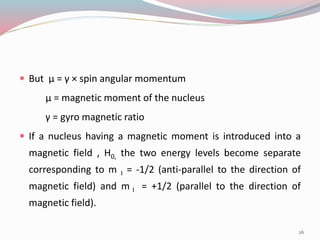
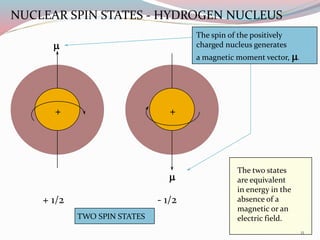
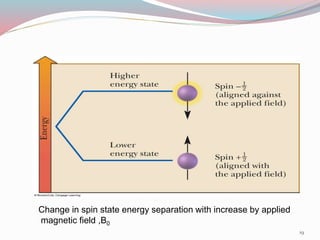
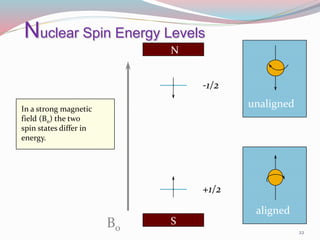
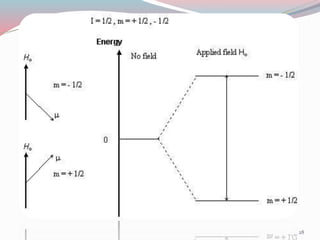
![ For a nucleus with I = 1/2, the energies E1 and E2 for the
two states with m I = +1/2 and m I = -1/2 , respectively,
are
E1 = -1/2 [ γ h / 2µ ] H0
E2 = + 1/2 [ γ h / 2µ ] H0
27](https://image.slidesharecdn.com/nmr-160315162509/85/Nuclear-Magnetic-Resonance-Spectroscopy-27-320.jpg)
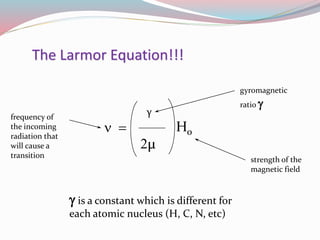
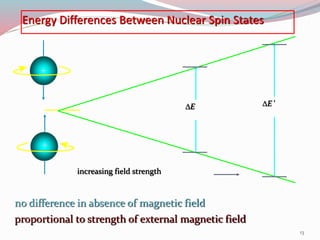
![ The frequency ν at which energy is absorbed or
emitted is given by Bohr̕ s relationship:
ν = E2 – E1 / h
ν = 1/2 [ γ h / 2µ ] H0 + 1/2 [ γ h / 2µ ] H0 / h ( or )
ν= γ / 2µ H0
This is the Larmor equation
30](https://image.slidesharecdn.com/nmr-160315162509/85/Nuclear-Magnetic-Resonance-Spectroscopy-30-320.jpg)