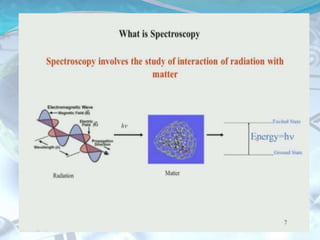
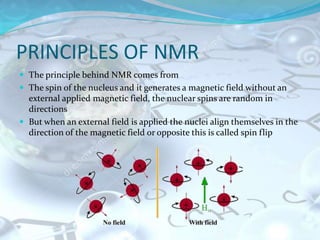
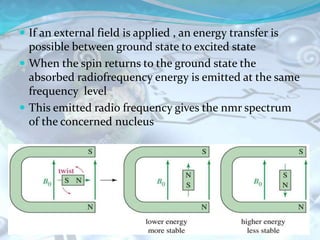
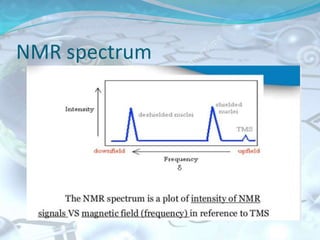
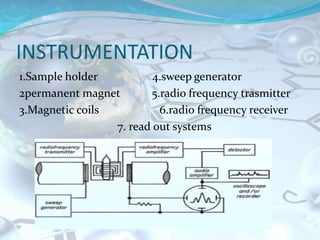
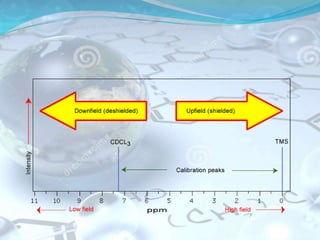
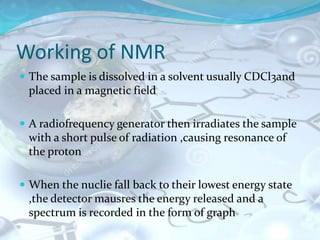
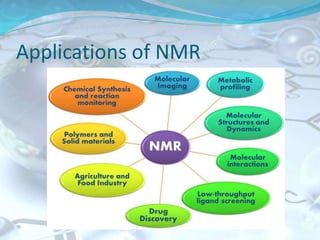
NMR spectroscopy is an analytical technique that uses the magnetic properties of certain nuclei, such as 1H and 13C, to characterize organic molecules. It was independently developed in the 1940s-1950s by groups at Harvard and Stanford, with Nobel Prizes awarded. There are two main types - 1H NMR studies hydrogen atoms and 13C NMR studies carbon atoms. The instrument uses a strong magnet to align nuclear spins, radio waves to excite them, and detectors to measure the radiofrequency energy emitted as the spins relax. NMR provides information about a molecule's structure through analysis of peak positions in its spectrum.