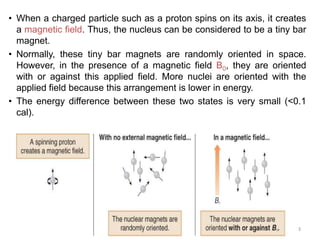
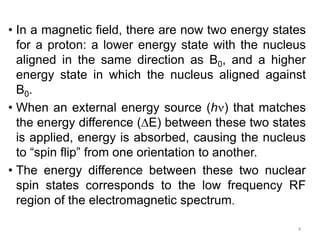
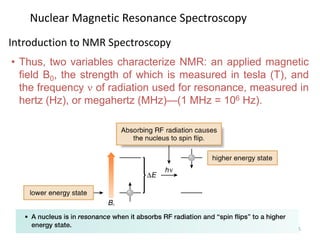
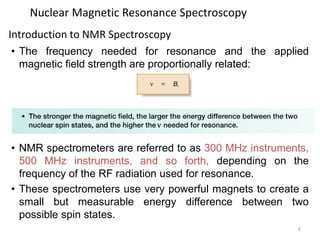
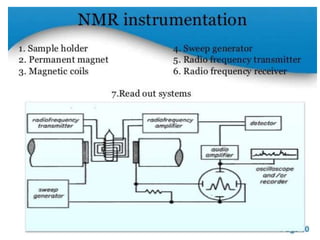
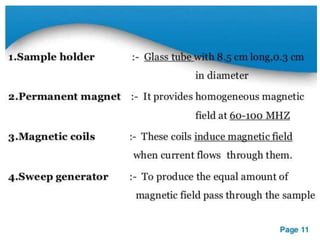
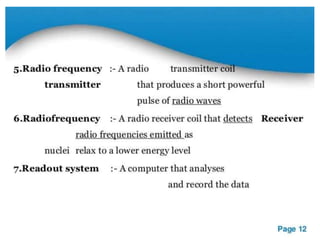
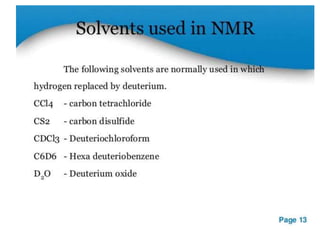
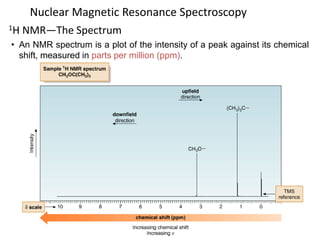
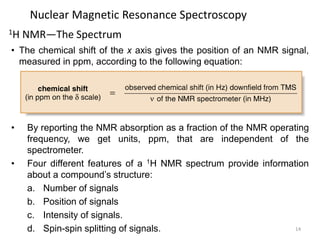
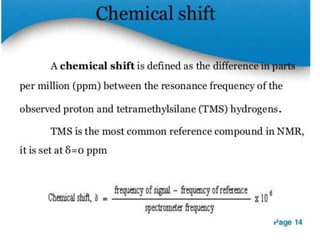
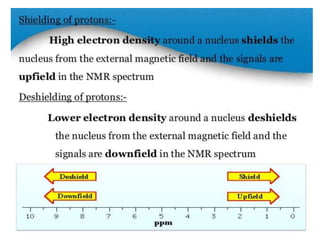
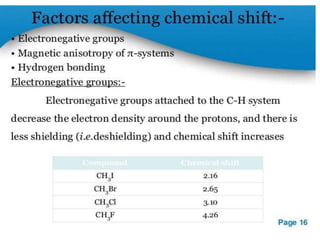
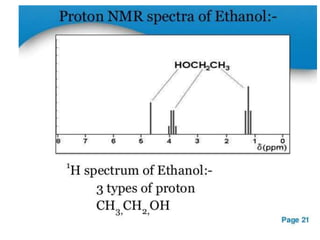
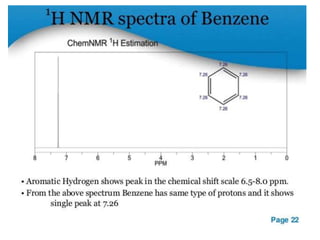
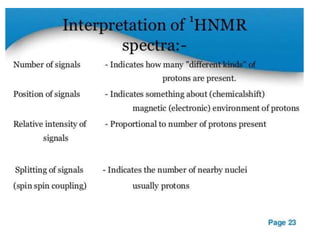
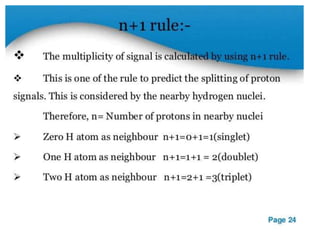
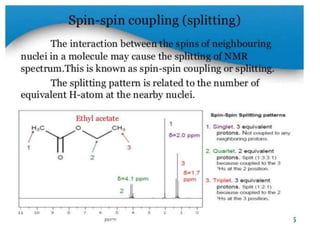
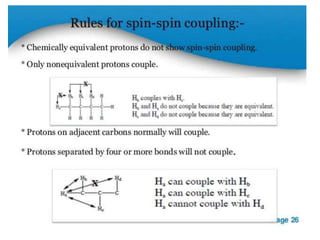
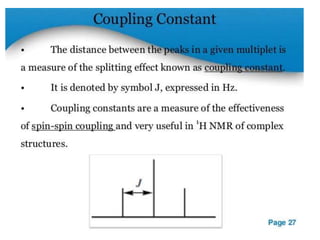
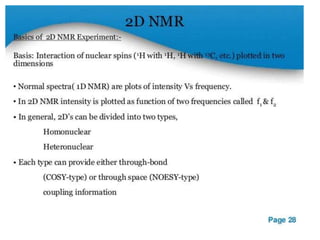
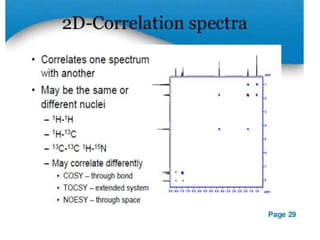
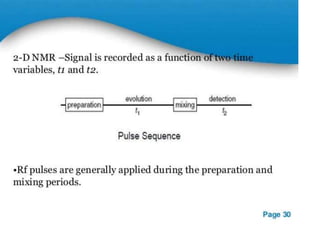
Nuclear magnetic resonance (NMR) spectroscopy uses radio waves to analyze organic molecules by determining their carbon-hydrogen frameworks. There are two main types of NMR: 1H NMR identifies hydrogen atoms and 13C NMR identifies carbon atom types. NMR works by placing the molecule in a strong magnetic field, which causes the nuclear spins of some elements to change orientations. When a matching radio wave is applied, energy is absorbed and the nucleus "spin flips" between energy states. The frequency at which this occurs depends on the molecule's electronic environment. NMR spectra plot peak intensity against chemical shift in parts per million to reveal structural information.