Embed presentation
Downloaded 498 times

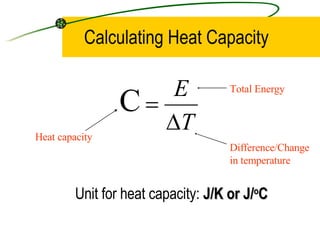

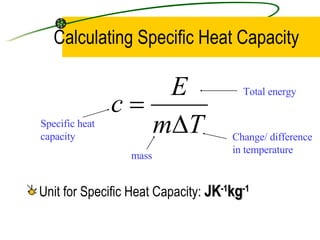

When an object is heated, its molecules absorb heat energy which increases their kinetic energy and causes them to move faster, raising the temperature of the substance. Heat capacity is defined as the amount of energy needed to raise the temperature of a substance by 1 degree Celsius or Kelvin, while specific heat capacity refers to the energy needed to raise the temperature of 1 kilogram of a substance by 1 degree. Specific heat capacity is calculated by dividing the total energy by the mass and temperature change.





