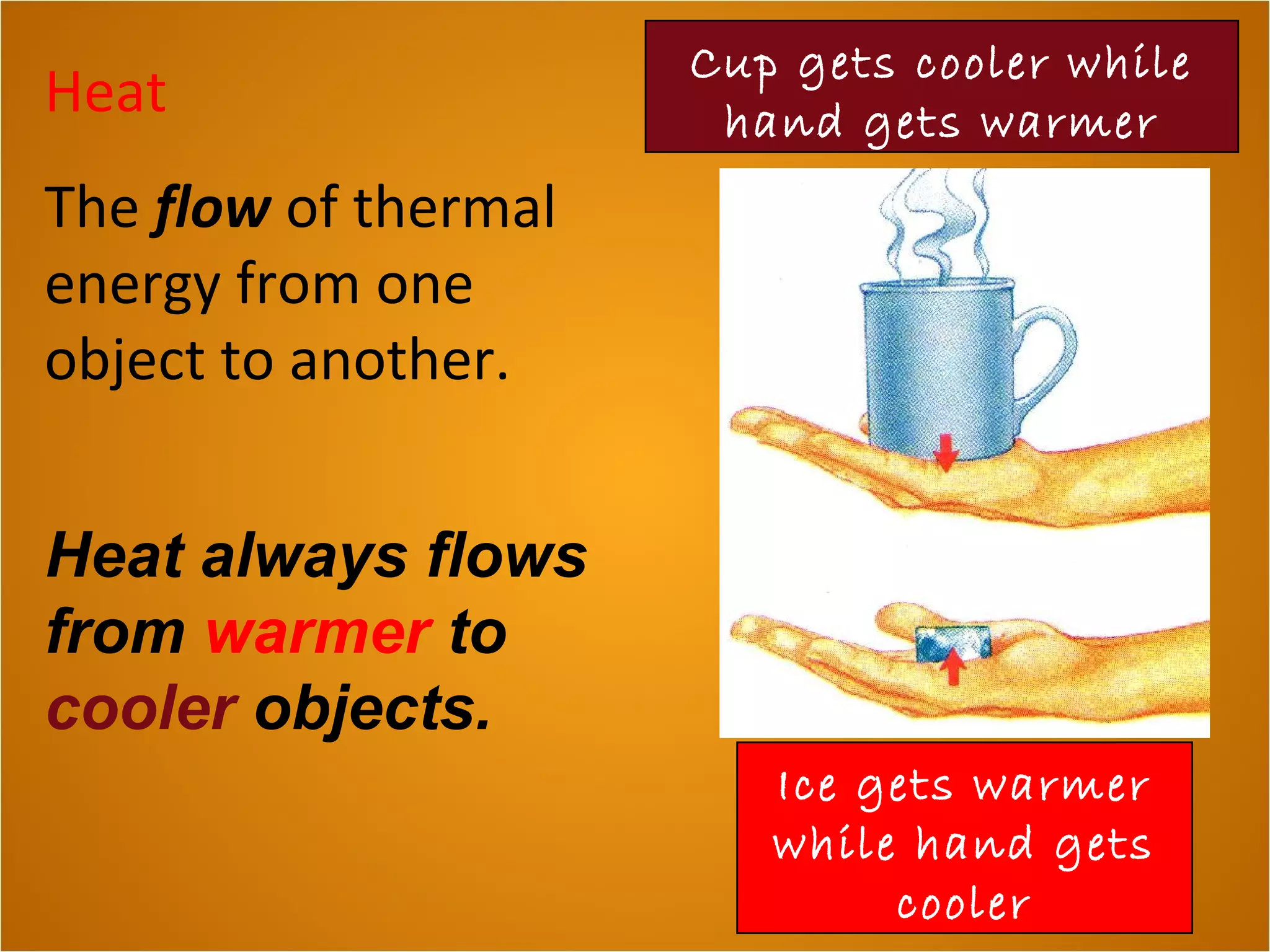
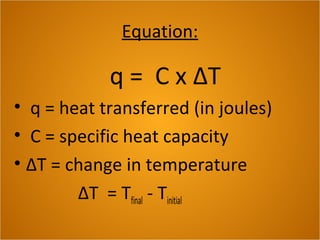
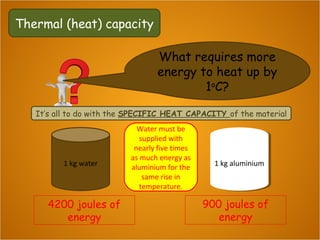
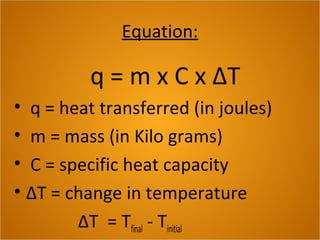
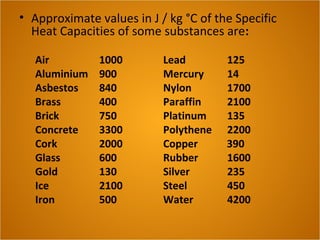
Heat is the flow of thermal energy from warmer objects to cooler ones. Different materials heat up and cool down at different rates because they have different specific heat capacities. The specific heat capacity is the amount of energy needed to raise the temperature of 1 kg of a material by 1°C. Materials with higher specific heat capacities, like water, require more energy to heat up the same amount compared to materials with lower specific heat capacities.