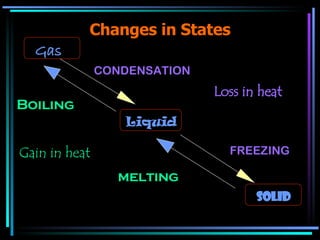
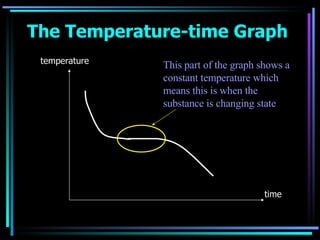
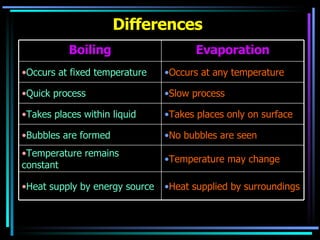
The document discusses the processes of melting, boiling, freezing and condensation. It explains that during these state changes, heat is absorbed or released in the form of latent heat, causing the temperature to remain constant. Impurities affect the freezing/melting and boiling points of substances by lowering or raising the temperature at which these state changes occur. Pressure also influences state changes, with higher pressure lowering melting/boiling points.