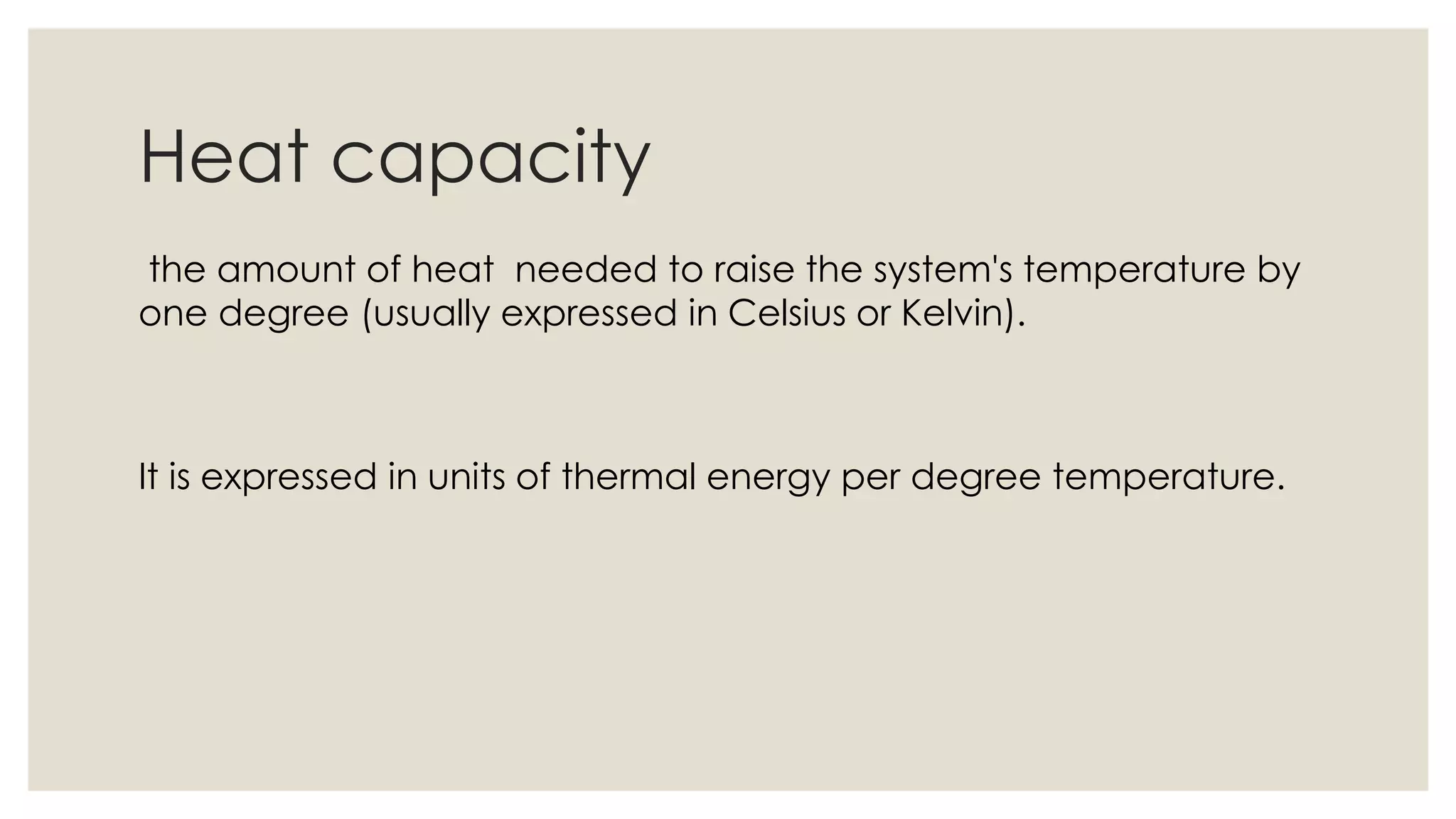
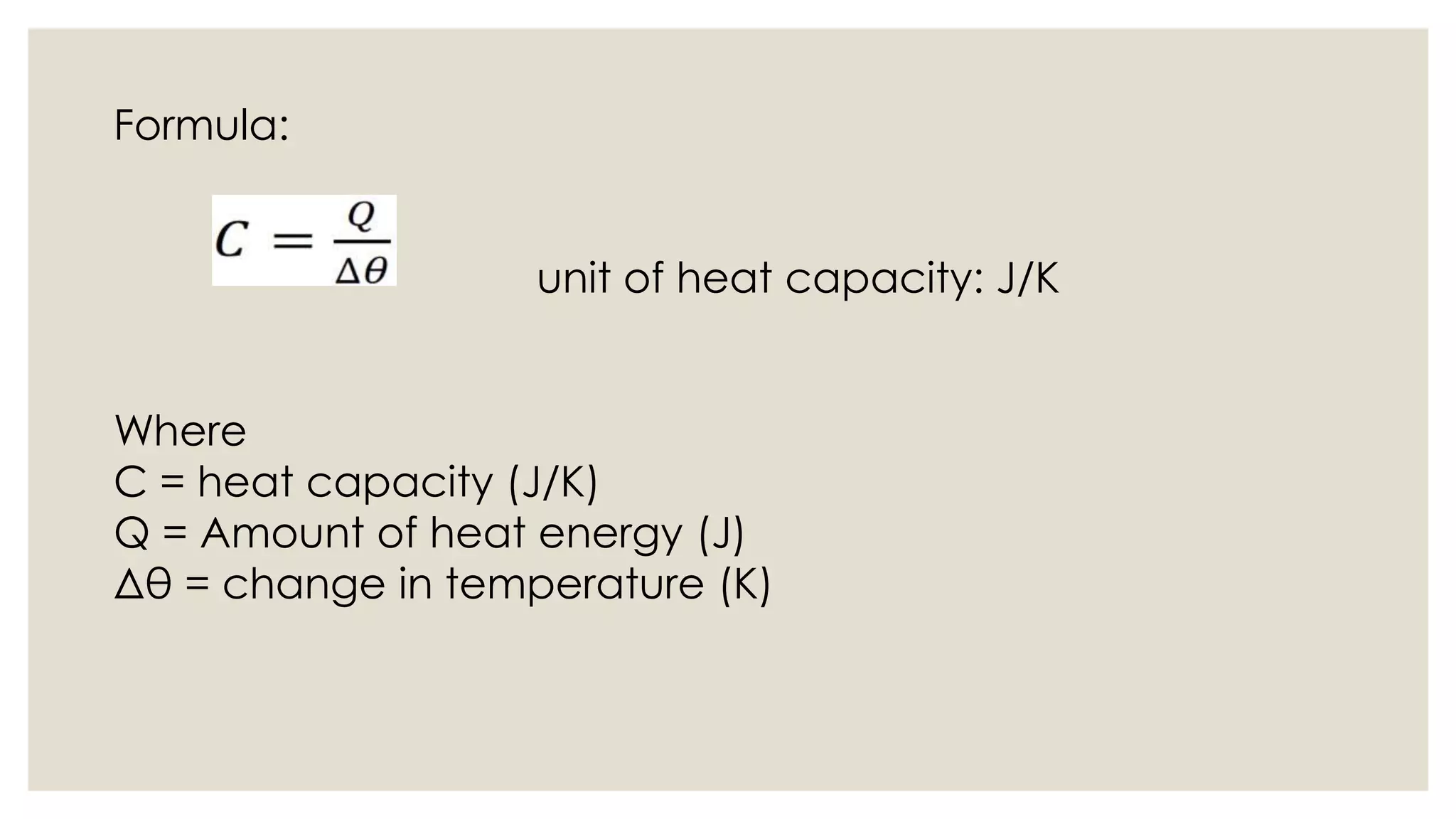
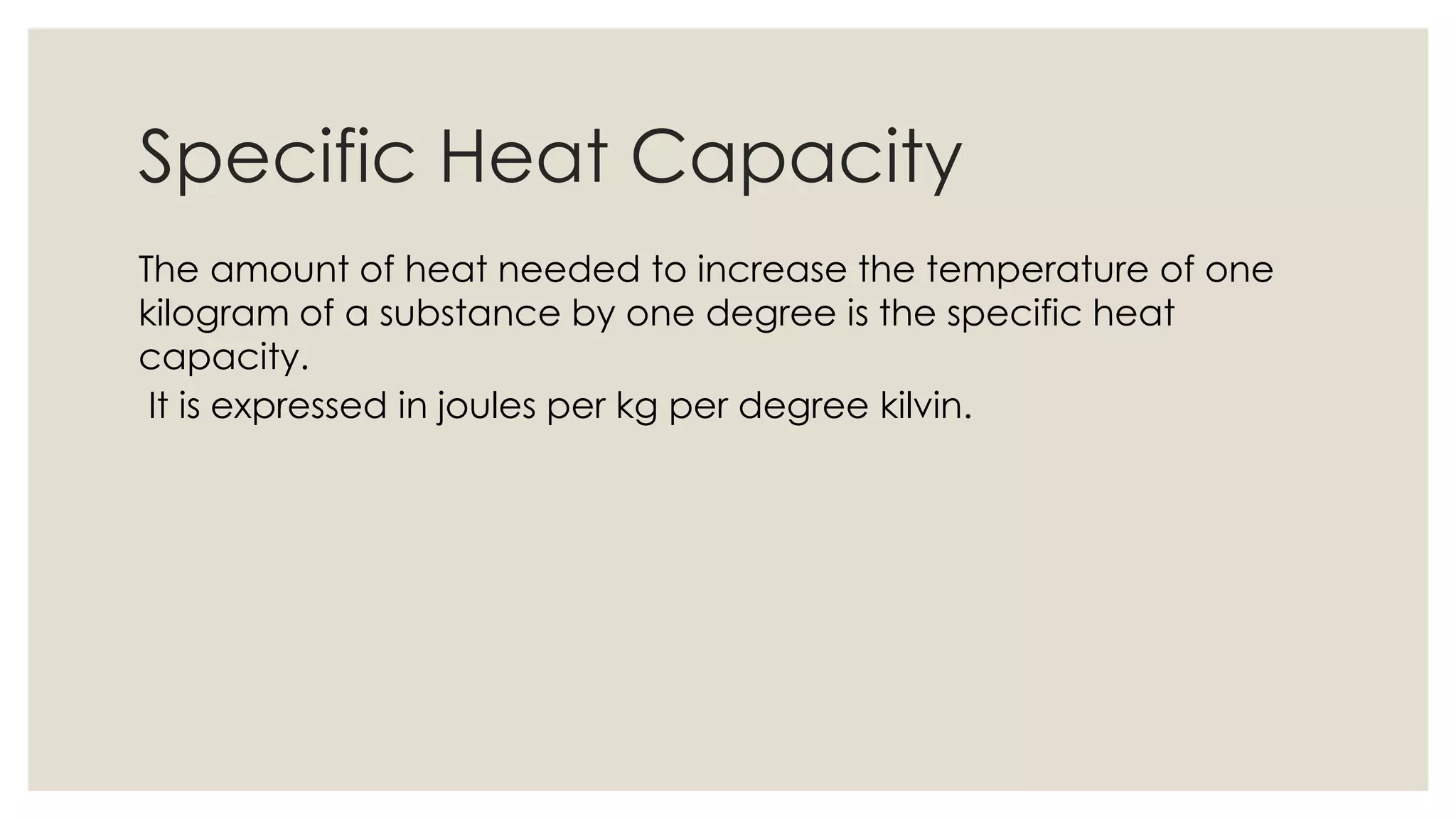
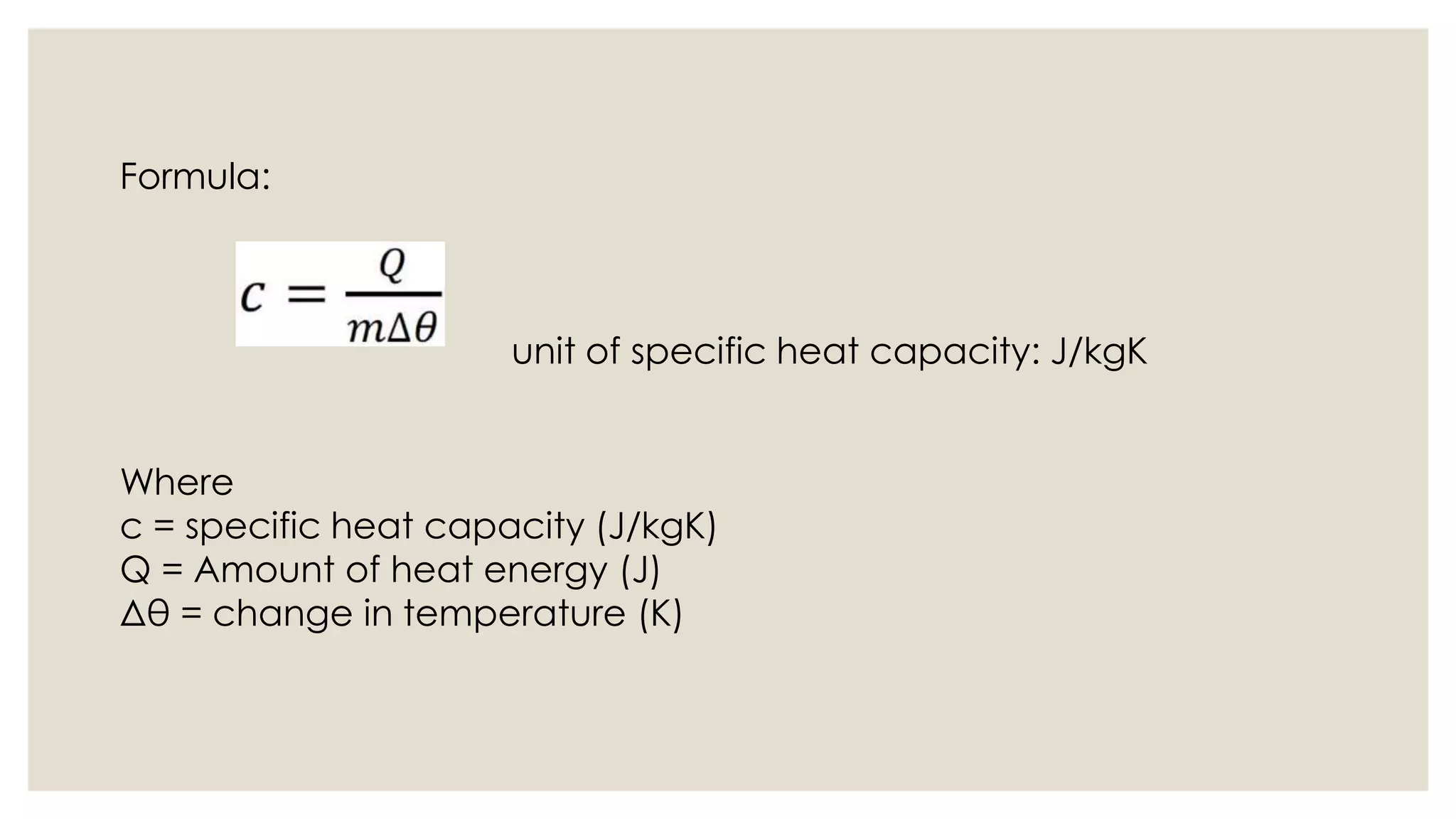
Heat capacity is the amount of heat needed to raise a system's temperature by one degree, expressed in units of thermal energy per degree. Specific heat capacity is the amount of heat needed to increase the temperature of one kilogram of a substance by one degree, expressed in joules per kg per degree Kelvin. The document provides formulas for heat capacity and specific heat capacity, and gives an example quiz to test understanding of specific heat capacity definitions and calculations involving changes in temperature and heat energy.