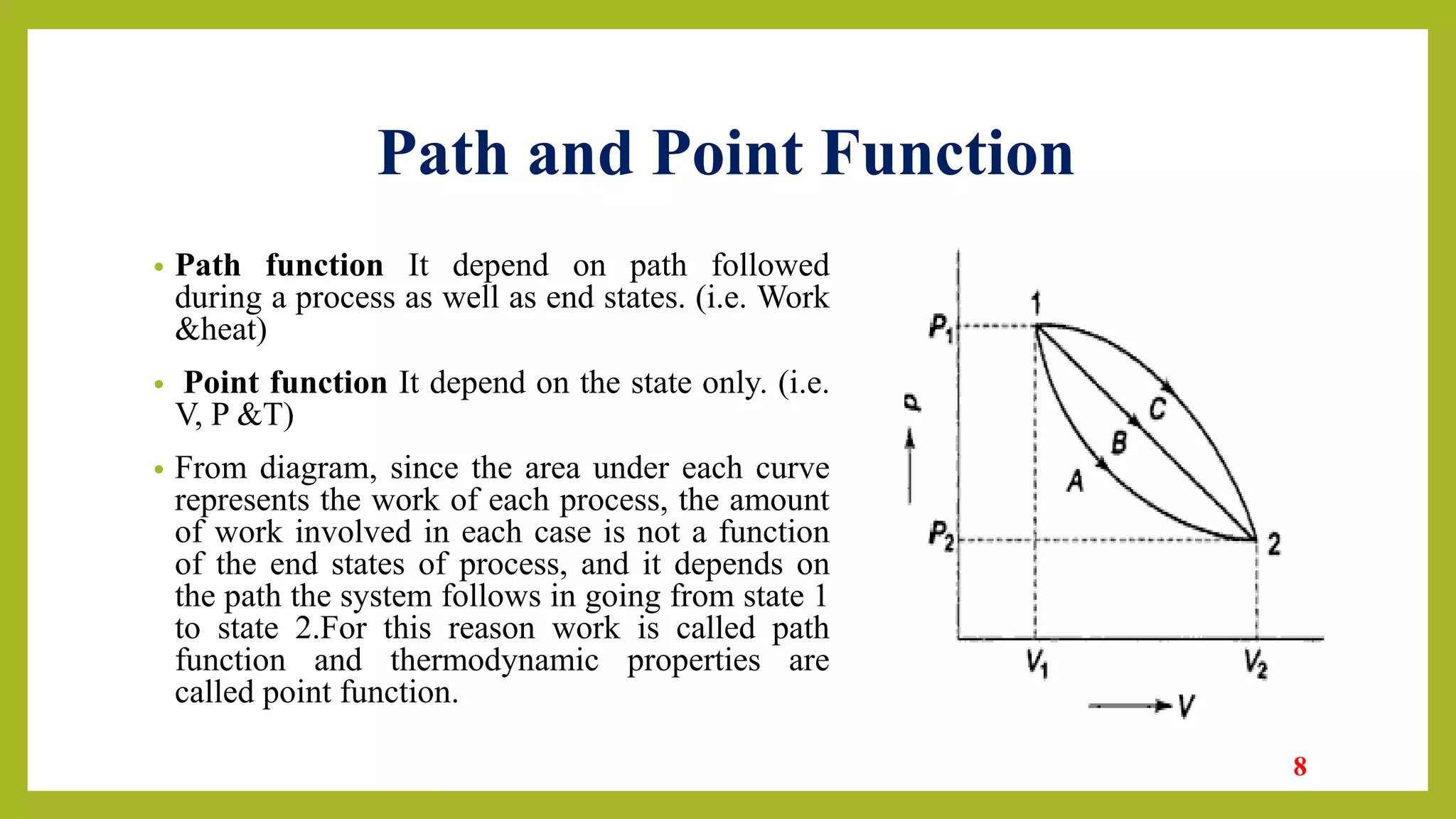
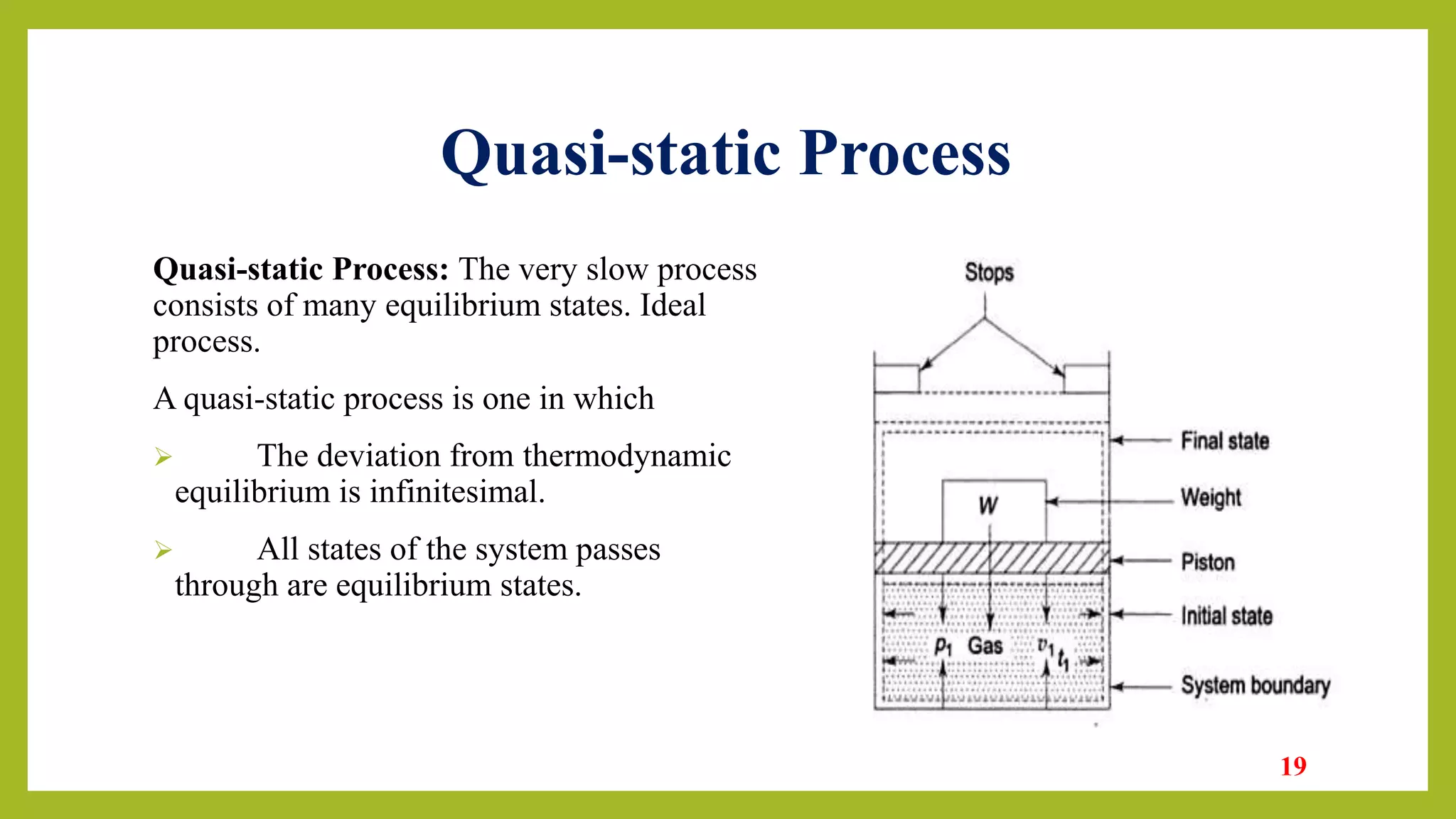
This document provides an introduction to thermodynamics. It defines thermodynamics as the science dealing with heat, work, and their relation to properties of matter and energy change. The document outlines the four laws of thermodynamics and describes the zeroth law regarding thermal equilibrium, the first law regarding conservation of energy and internal energy, the second law regarding limits on heat conversion and direction of processes, and the third law defining absolute zero entropy. Examples of engineering applications are given in areas like heat engines, refrigeration, and air conditioning. Key concepts discussed include system, surroundings, state, path, process, equilibrium, intensive/extensive properties, and reversible/irreversible processes.