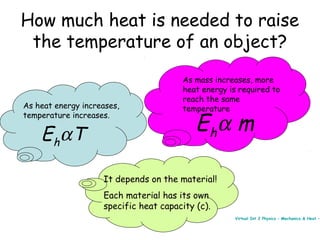
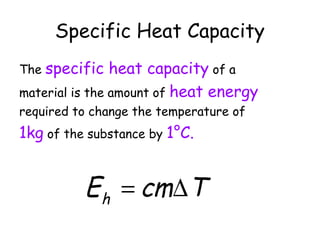
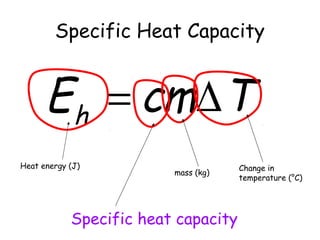
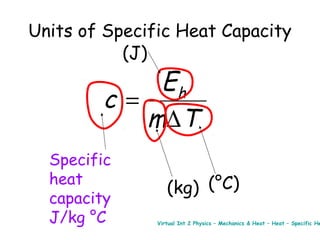
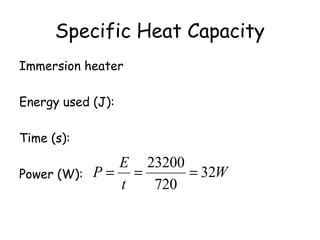
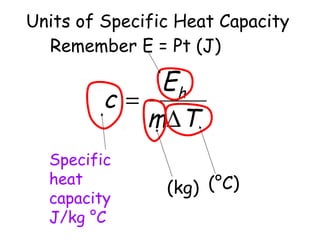
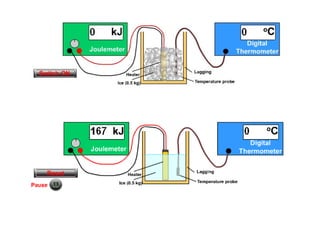
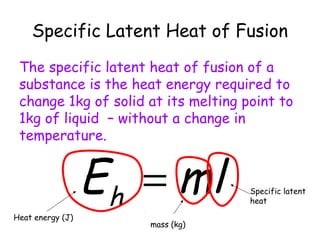
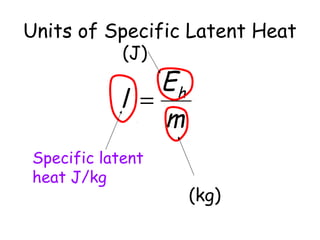
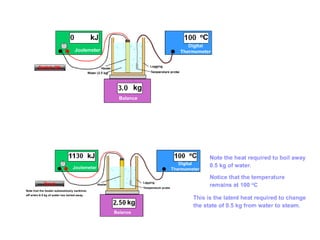
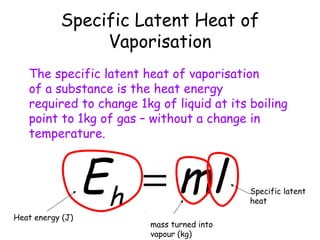
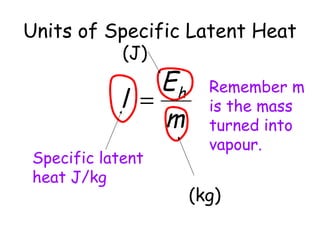
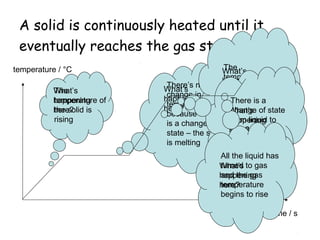
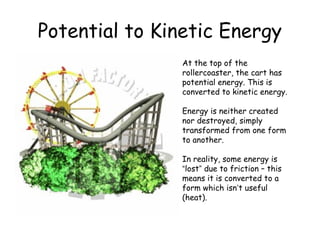
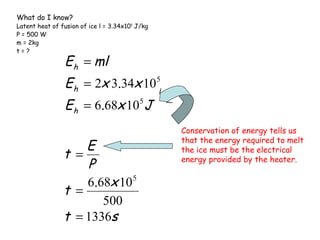
This document discusses key concepts in thermal physics including heat, temperature, specific heat capacity, and latent heat. It begins by defining heat as a form of energy and temperature as a measurement of how hot or cold something is. It explains that different materials require different amounts of heat to change temperature by the same amount due to differences in specific heat capacity. The document then discusses phase changes and how heat is required for changes of state, like melting and boiling, without a change in temperature due to the absorption of latent heat. It provides examples of calculating specific heat capacity and using the principle of conservation of energy to solve problems involving heat transfer.