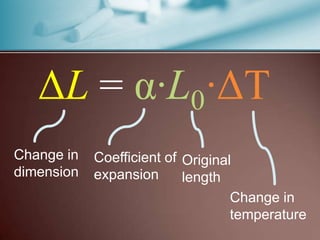
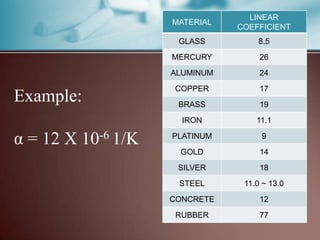
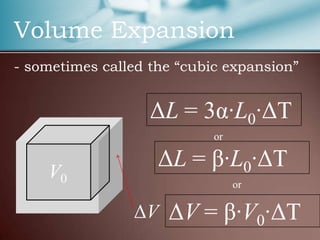
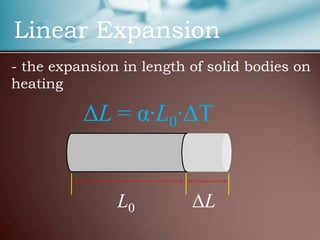This document discusses thermal expansion, which is the change in dimensions of materials due to changes in temperature. It defines linear expansion as the expansion in length and volume expansion as expansion in overall volume. The key factors that affect thermal expansion are the temperature change, material type, and original dimensions. Common materials and their coefficients of thermal expansion are provided. Examples of applications like loosening tight jar lids are given. Finally, several problems involving calculating dimensional changes due to temperature changes are presented.