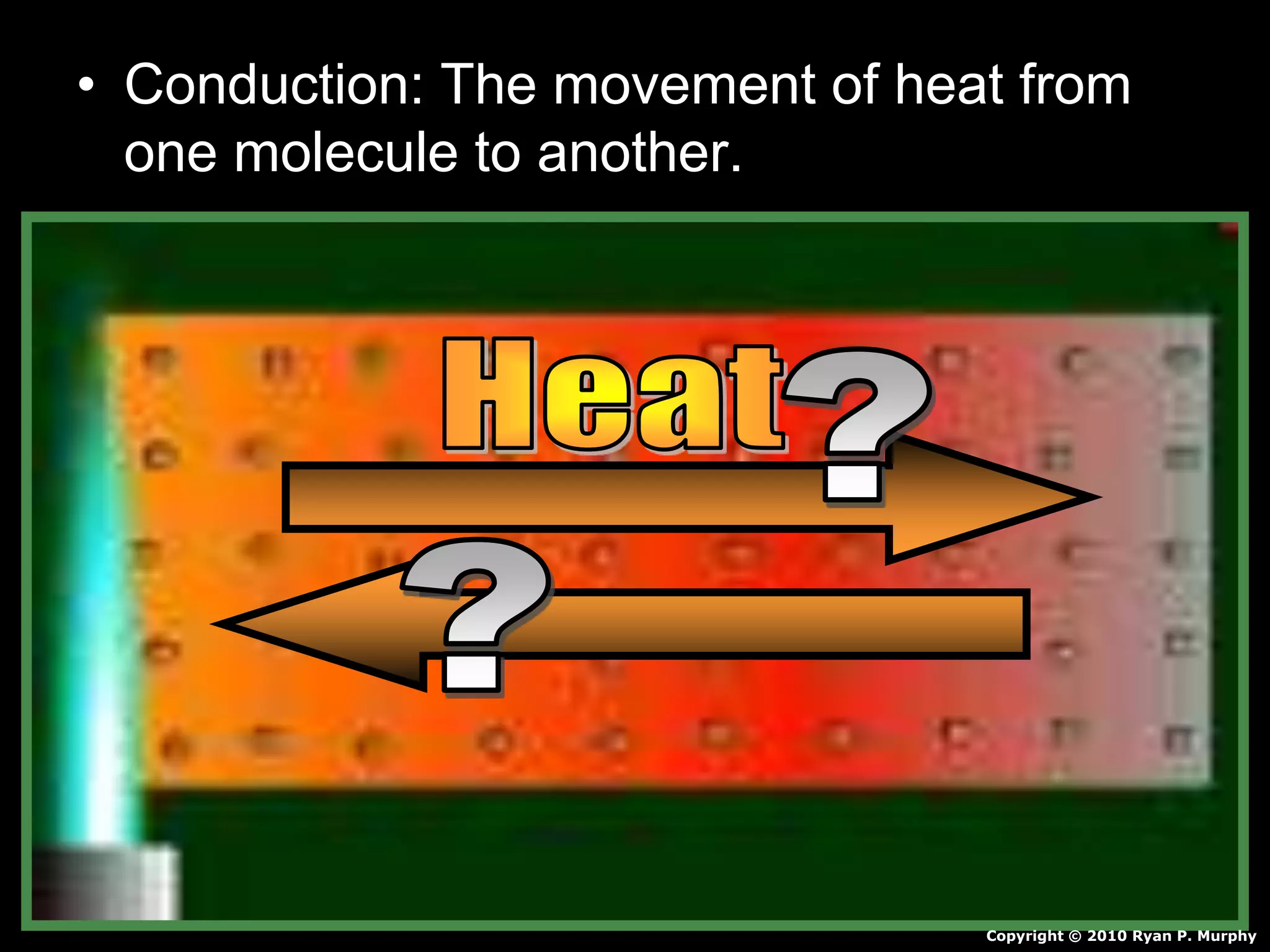
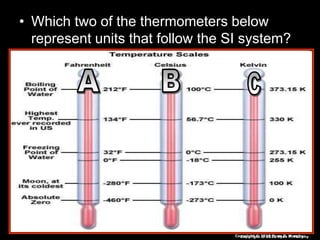
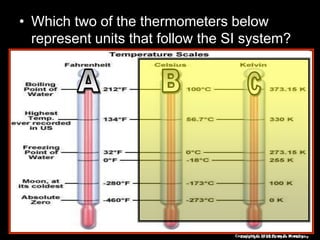
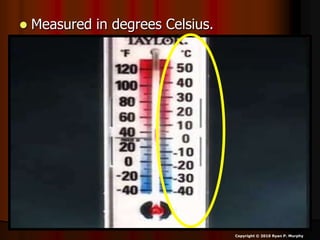
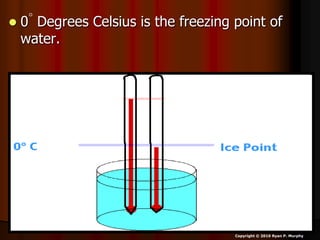
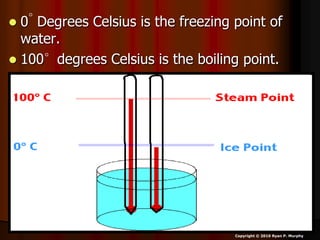
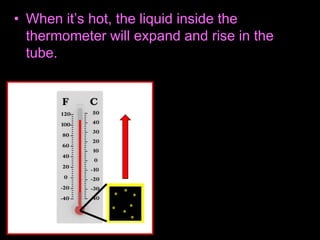
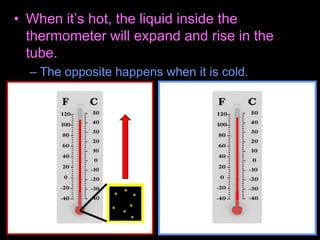
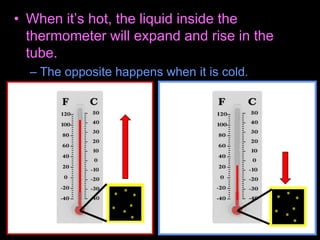
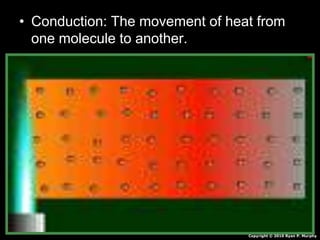
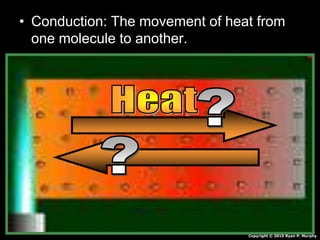
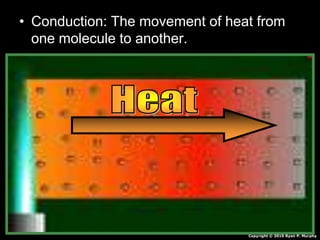
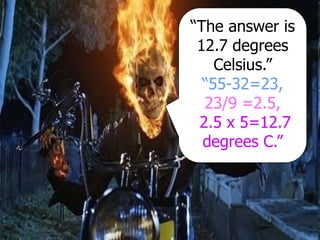This document appears to be a science lesson plan on the topic of temperature and conduction. Some key points covered include:
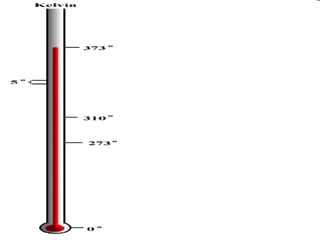
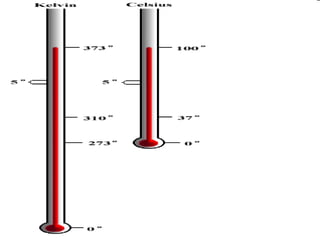
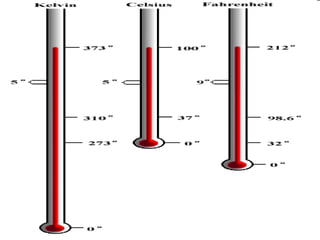
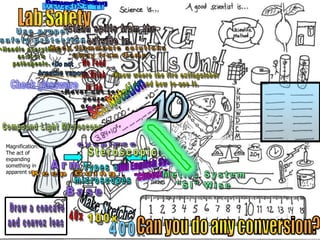
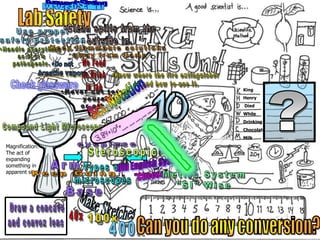
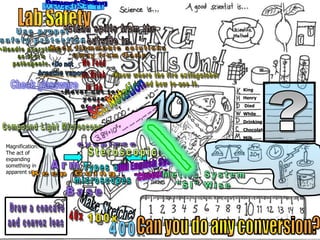
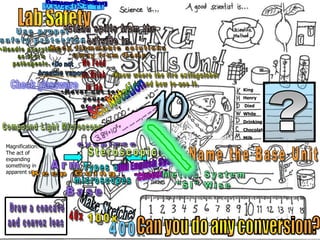
- Definitions of conduction, temperature, and different temperature scales such as Celsius, Fahrenheit, Kelvin.
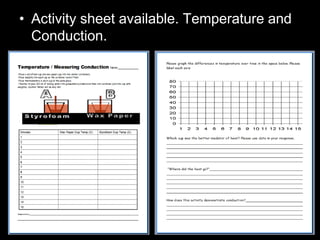
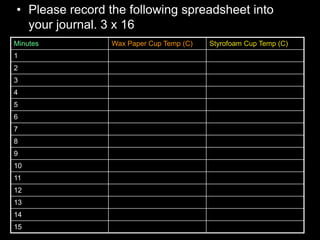
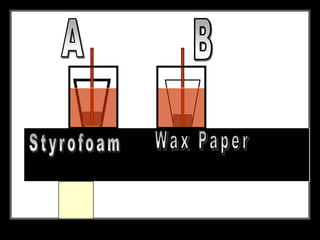
- An activity is described where students measure the temperature change over time of water in styrofoam and wax paper cups, demonstrating conduction.
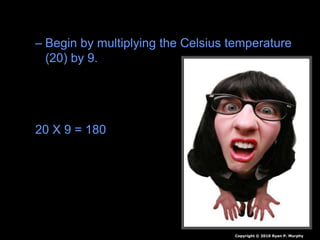
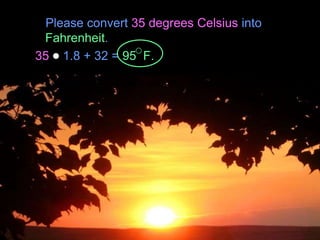
- Conversions between Celsius and Fahrenheit temperatures are practiced.
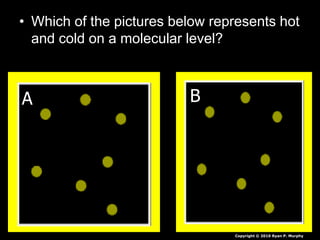
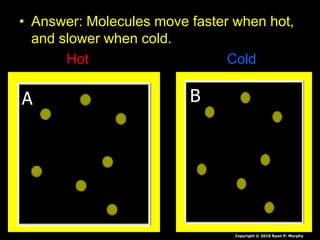
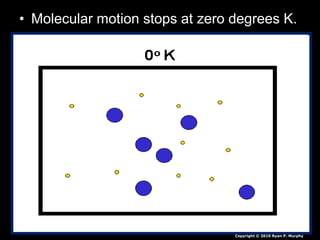
- Additional topics covered are molecular motion and temperature, different types of thermometers, and an optional "Red Light, Green Light" activity related to temperature.