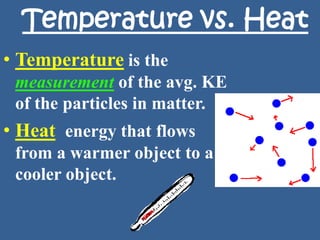
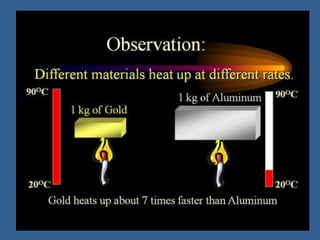
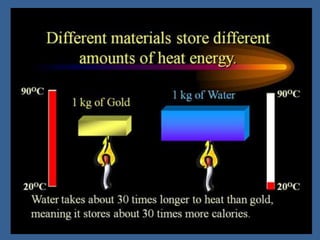
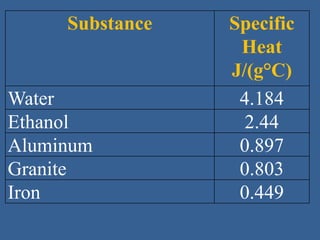
This document discusses specific heat capacity and how it relates to heat transfer and temperature change. Specific heat capacity is the amount of heat required to raise the temperature of 1 gram of a substance by 1 degree Celsius. Water has a relatively high specific heat of 4.184 J/g°C, while other substances like aluminum (0.897 J/g°C) and iron (0.449 J/g°C) have lower specific heat capacities. The specific heat capacity of a substance determines how quickly or slowly it will heat up when heat is added. The document also provides an equation to calculate the heat absorbed or released given the specific heat, mass, and temperature change of a substance.