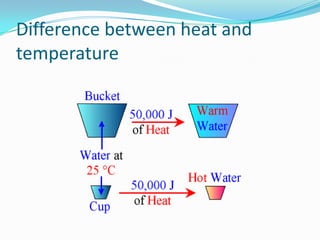
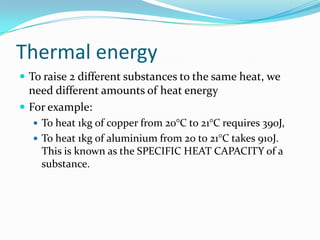
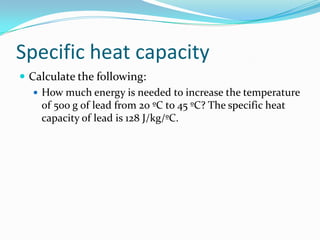
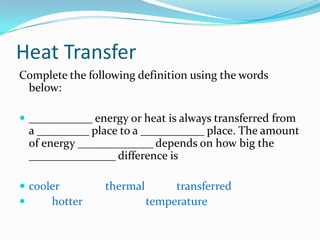
Thermal energy, also called heat energy, is the transfer of energy between objects due to a temperature difference. Temperature is measured in degrees Celsius and indicates how hot an object is, while heat is measured in Joules and refers to the quantity of thermal energy transferred. The specific heat capacity of a substance is the amount of heat energy required to raise its temperature by one degree and one kilogram, and can be used to calculate the energy needed to change an object's temperature. Heat naturally transfers from hotter to cooler objects until they reach thermal equilibrium.