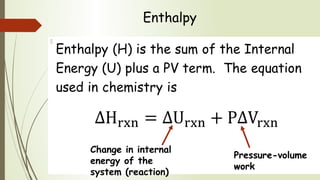
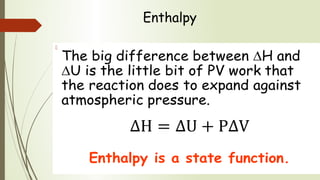
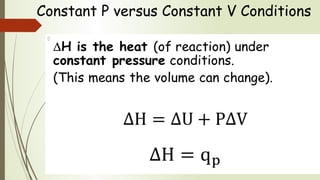
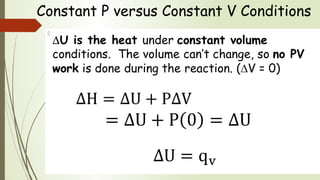
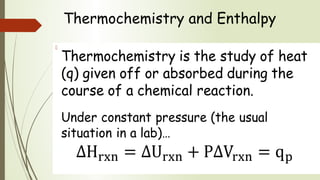
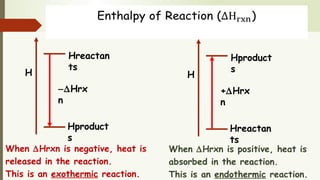
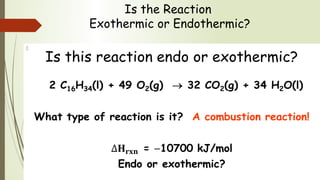
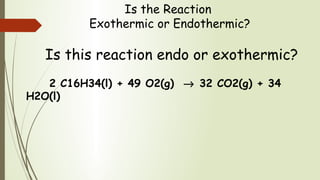Enthalpy (H) is the sum of internal energy (U) plus pressure-volume work. ΔH is the heat of reaction under constant pressure, while ΔU is the heat under constant volume. Thermochemistry studies the heat absorbed or released during chemical reactions. Combustion reactions are exothermic, releasing heat. Enthalpy is a state function that can be used to determine if a reaction is exothermic or endothermic based on the sign of the enthalpy change.