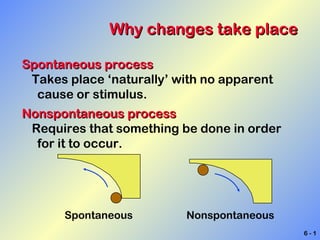
Spontaneous processes occur naturally without an external stimulus, while nonspontaneous processes require something to be done to occur. Whether a reaction is spontaneous can be determined using thermodynamics by calculating the enthalpy and entropy. Energy exists in various forms including thermal, electrical, chemical, and kinetic, and it can be transferred or changed between objects and forms. During chemical reactions, energy is either absorbed or released as bonds break and form.