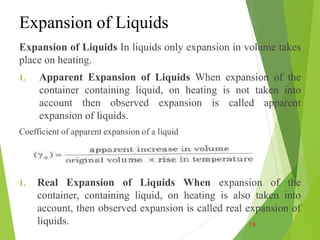
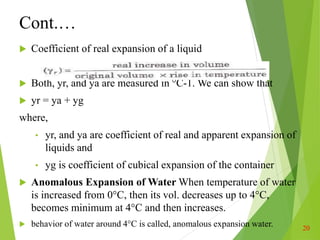
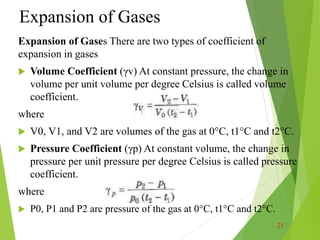
Thermal properties of materials including heat capacity, thermal expansion, conductivity, and stresses are important considerations in engineering. Heat capacity is a measure of how much energy is required to change a material's temperature. Thermal expansion describes how a material increases in size with increasing heat. Thermal conductivity determines how quickly heat transfers through a material. Thermal stresses occur due to temperature differences and constraints, which can cause cracking or deformation. Understanding these thermal properties aids engineering design for withstanding various temperature conditions and stresses.


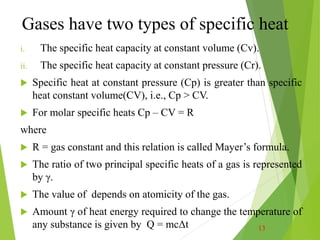
![Heat capacity
Heat capacity is a material’s ability to absorb heat from the
external surroundings; it represents the amount of energy
needed to increase the temperature of a substance 1 degree, so
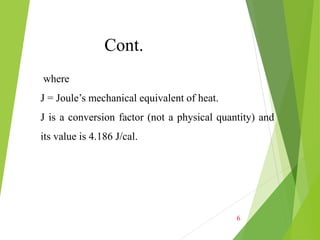
the units are J / oC. In mathematical terms, the heat capacity C
is expressed as follows: C = ΔQ/ΔT = dQ/dT [J/deg] Where
dQ is the energy required to produce a dT temperature change.
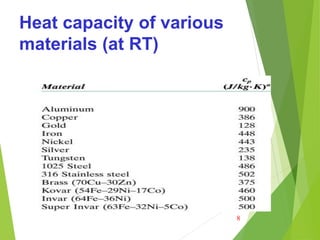
Ordinarily, heat capacity is specified per mole of material (e.g.,
J/mol-K, or cal/mol-K). Table below give the heat capacity of
some materials. When the temperature is increased, the kinetic
energy of the particles in the material changes. 7](https://image.slidesharecdn.com/presentation1-230808110818-7804c336/85/Presentation1-pptx-7-320.jpg)

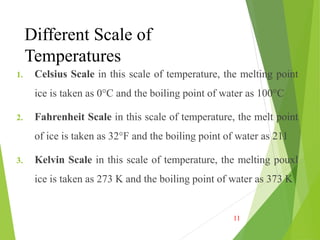
![Relation between Different Scales of
Temperatures
Specific Heat
The amount of heat required to raise the temperature of unit
mass the substance through 1°C is called its specific heat.
It is denoted by c or s.
Its SI unit is joule/kilogram-°C'(J/kg-°C).
Its dimensions are [L2T-2θ-1].
The specific heat of water is 4200 J kg-1°C-1 or 1 cal. g-1
C-1, which high compared with most other substances.
12](https://image.slidesharecdn.com/presentation1-230808110818-7804c336/85/Presentation1-pptx-12-320.jpg)