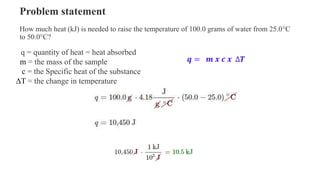
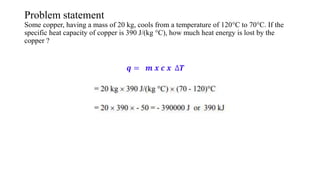
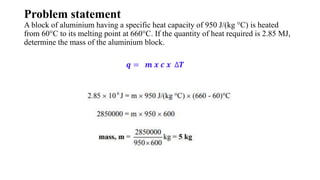
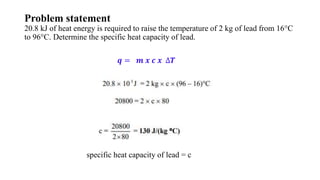
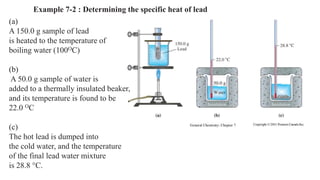
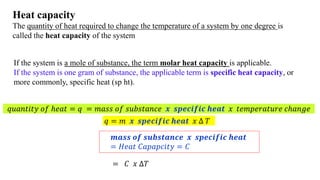
The document discusses heat, thermal energy, and heat capacity, defining key terms such as system, surroundings, kinetic energy, and potential energy. It explains the concepts of specific heat capacity and molar heat capacity, along with formulas for calculating heat transfer based on mass and temperature change. Several problem statements are provided to illustrate the application of these concepts in real-world scenarios.


![the Specific heat of 𝐶 𝑤𝑎𝑡𝑒𝑟 = 4.18
𝐽
𝑂 𝐶 𝑔 − 𝑤𝑎𝑡𝑒𝑟
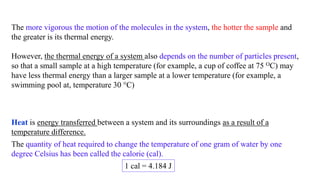
a substance's specific heat tells you how much heat is needed in order to increase the
temperature of 1 g of that substance by 1OC.
Now let's say that you wanted to cause a
1∘C increase in a 2-g sample of water
a 1∘C increase in the temperature of
m grams of water,
To increase the temperature of m g of water by [ n OC ], you'd need to supply it with
𝐻𝑒𝑎𝑡 𝑟𝑒𝑞𝑢𝑖𝑟𝑒𝑑 = 𝑚 𝑥 𝑛 𝑥 𝑠𝑝𝑒𝑐𝑖𝑓𝑖𝑐 ℎ𝑒𝑎𝑡
= 𝑚 𝑥 𝑠𝑝𝑒𝑐𝑖𝑓𝑖𝑐 ℎ𝑒𝑎𝑡 𝑥 𝑛
𝐻𝑒𝑎𝑡 𝑟𝑒𝑞𝑢𝑖𝑟𝑒𝑑 = 𝑚 𝑥 𝑆𝑝𝑒𝑐𝑖𝑓𝑖𝑐 ℎ𝑒𝑎𝑡 𝑥 ∆ 𝑇
𝒒 = 𝒎 𝒙 𝒄 𝒙 ∆𝑻](https://image.slidesharecdn.com/heatandheatcapacity-200416125610/85/Heat-and-heat-capacity-7-320.jpg)