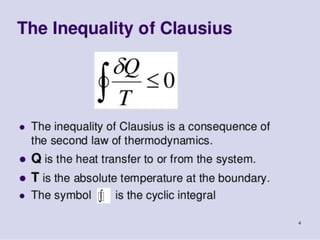
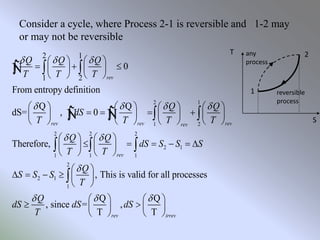
The document discusses entropy, which is a thermodynamic property that serves as a tool for analyzing engineering devices according to the second law of thermodynamics. Entropy can be viewed as a measure of disorder in a system, with more disorganized systems having higher entropy. The Clausius inequality and definition of entropy using heat transfer and temperature are provided. The principles of entropy change for open and closed systems, as well as the increase of entropy principle and third law of thermodynamics, are summarized.