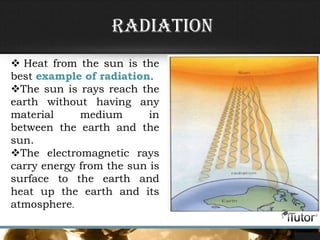
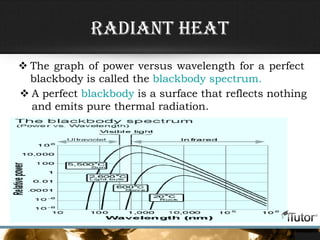
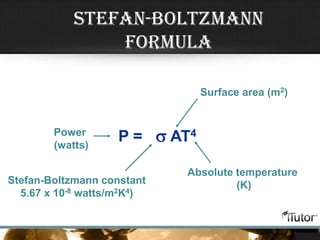
This document defines key concepts related to heat transfer including:
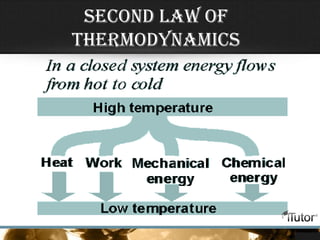
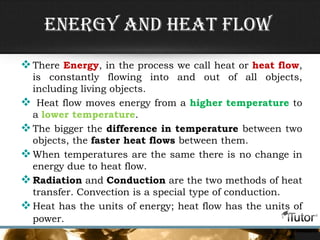
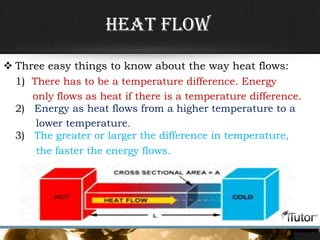
- Heat is the transfer of energy between objects due to temperature differences and is measured in Joules.
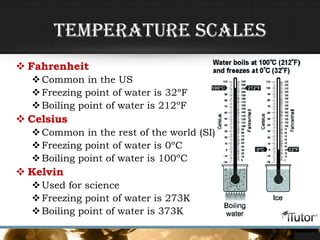
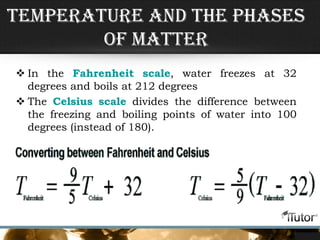
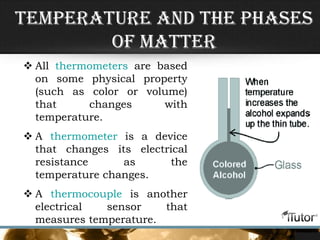
- Temperature is determined by the motion of particles in a substance, with faster motion indicating a higher temperature.
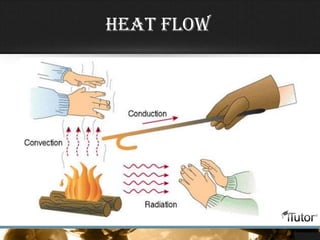
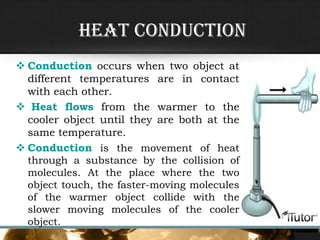
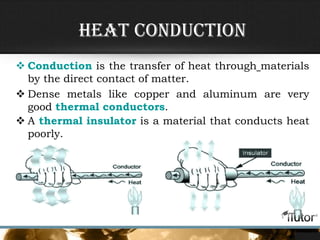
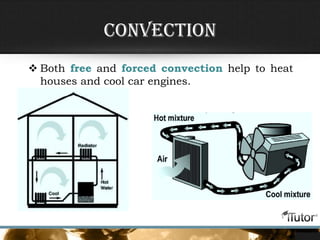
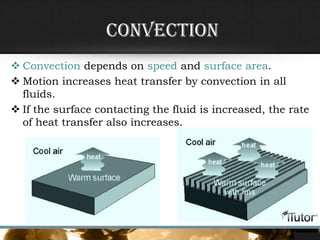
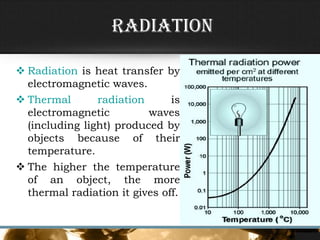
- The three primary methods of heat transfer are conduction, convection, and radiation. Conduction involves direct contact, convection involves the transfer of heat by a liquid or gas, and radiation involves the transfer of heat through electromagnetic waves.
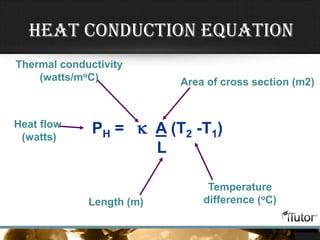
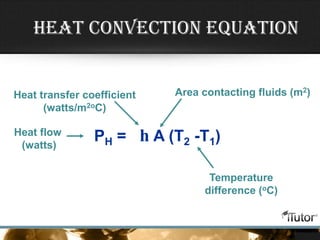
- Equations are provided to calculate heat transfer via conduction, convection, and radiation based on factors like thermal conductivity, heat transfer coefficient, surface area, and temperature differences.