This document discusses the three main processes of heat transfer: conduction, convection, and radiation.
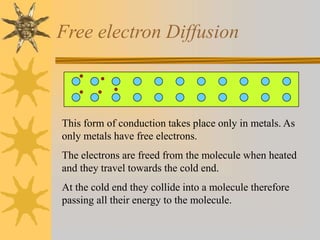
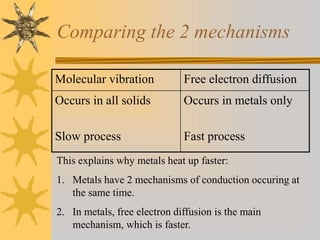
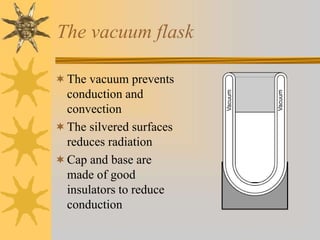
Conduction involves the transfer of thermal energy through direct contact of molecules in solids. Convection occurs through the circulation of liquids and gases, such as heated water rising. Radiation transfers heat through electromagnetic waves and does not require a medium. Good conductors readily transfer heat through conduction while insulators inhibit heat transfer. The processes spread heat from hot to cold regions through molecular vibration, fluid circulation, and electromagnetic radiation.