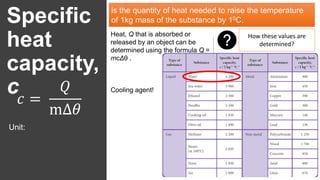
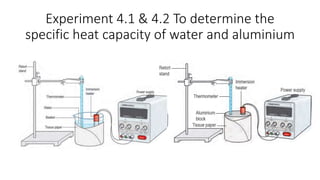
The document discusses the concept of specific heat capacity, illustrating its importance with examples from various contexts such as cooking and material selection in engineering. It includes definitions, formulas for calculating heat transfer, and practical problems related to specific heat capacity. Additionally, it outlines activities for exploring real-life applications and encourages collaborative projects to address temperature issues in building designs.













![Quiz 1. What is the difference between heat capacity
and specific heat capacity?
2. How much heat energy is needed to increase
the temperature of a 0.2 kg mass of gold
by 10°C?
[Given the value of specific heat capacity of
gold is 300 J kg–1 °C–1]](https://image.slidesharecdn.com/2specificheatcap-210131140652/85/2-specific-heat-cap-16-320.jpg)