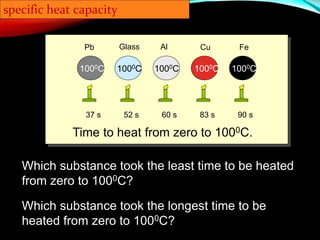
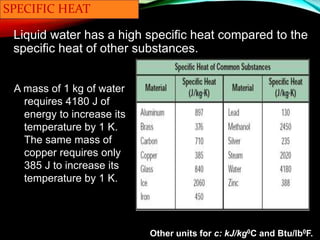
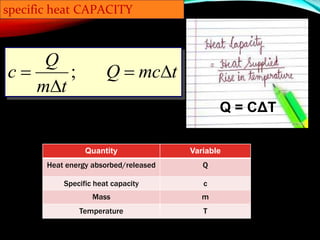
Specific heat capacity is a measurement of the amount of heat required to change the temperature of a substance. It is defined as the amount of heat needed to raise the temperature of 1 kilogram of a substance by 1 degree Celsius. Substances with high specific heat capacities, like water, require more heat to change their temperature compared to substances with lower specific heat capacities. The document provides examples of how long it takes different materials to heat up by 1000°C when given the same amount of heat. It also provides the specific heat capacity equation and examples of how to calculate the heat required to change a substance's temperature.