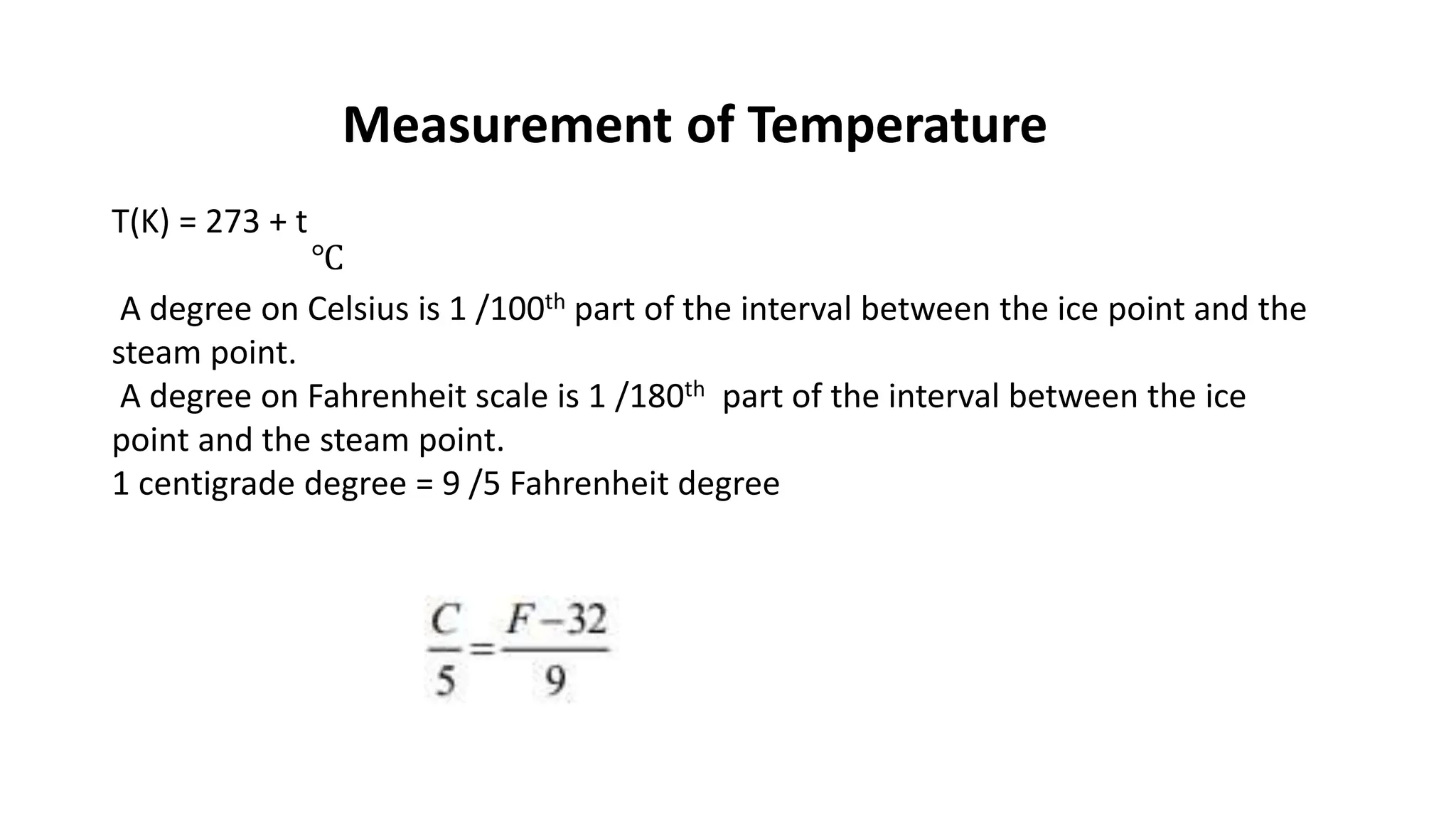
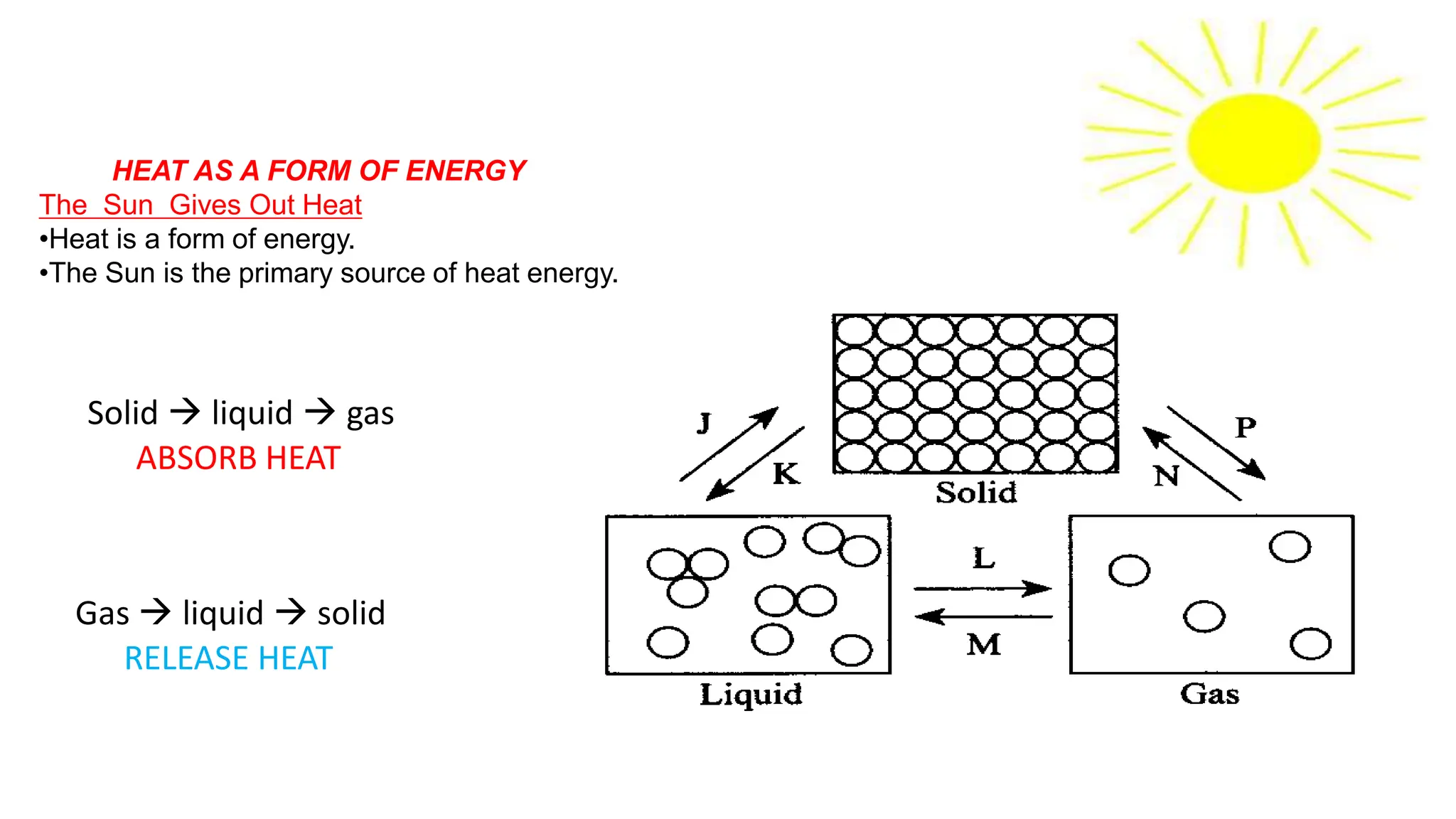
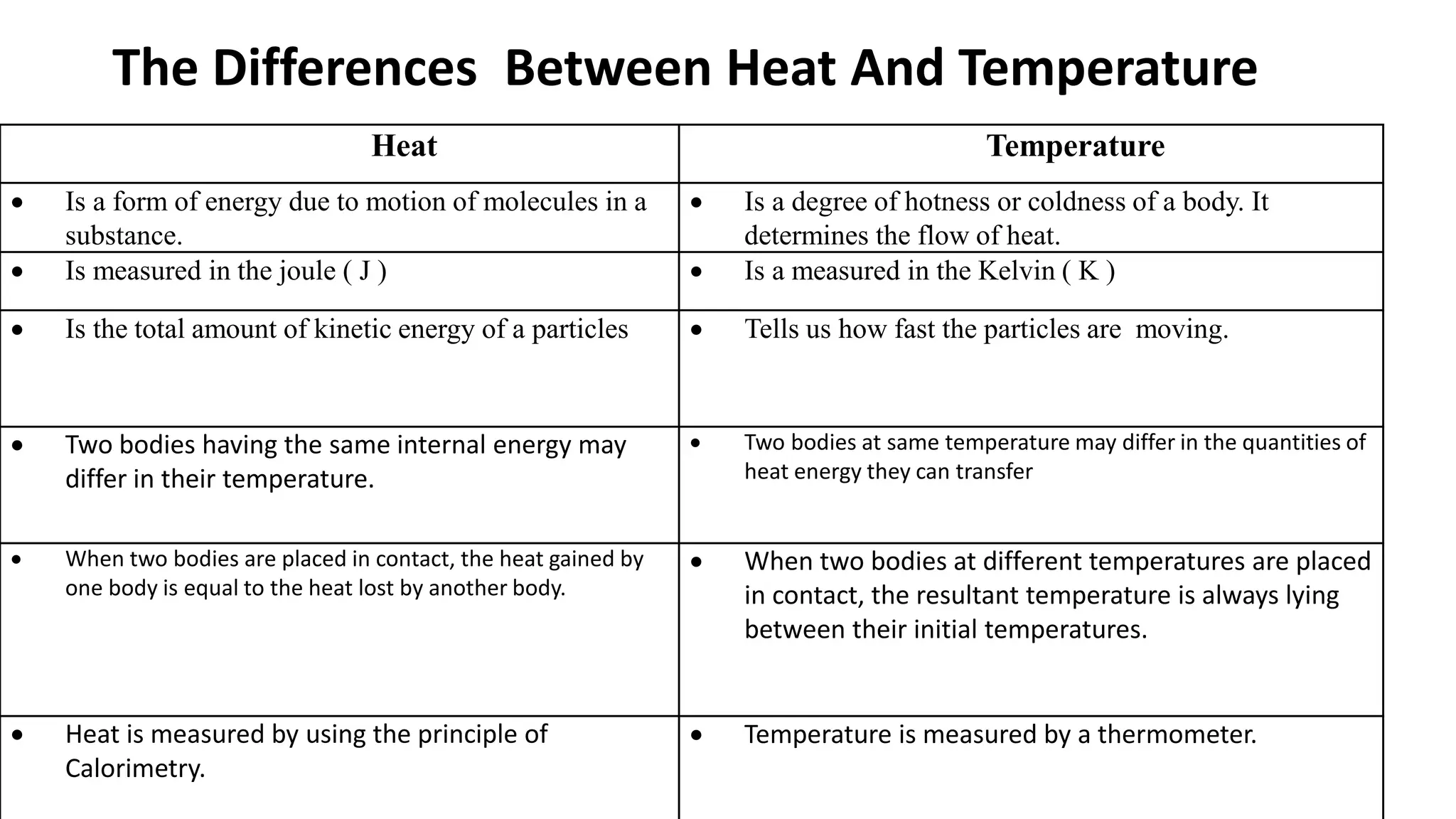
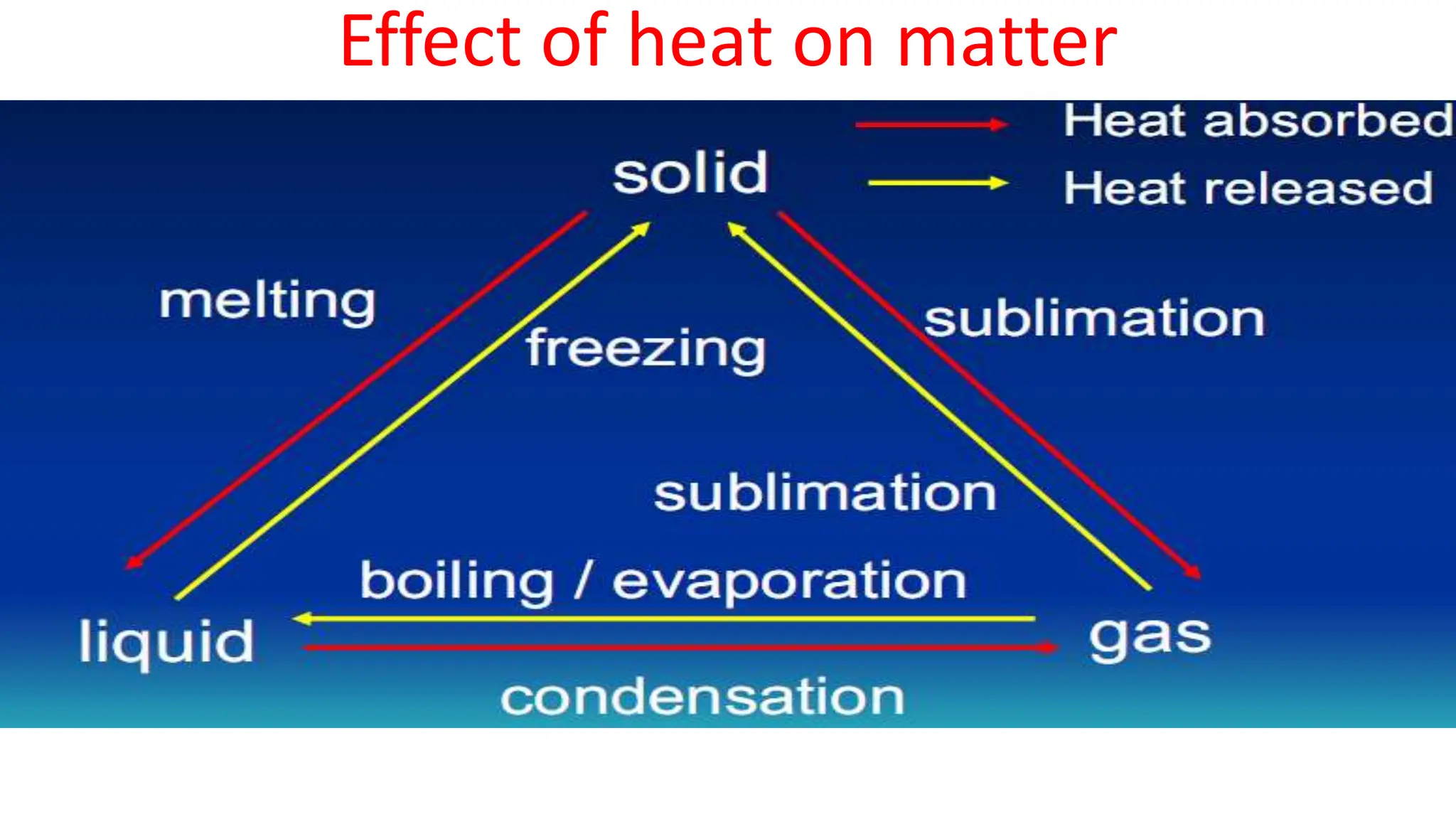
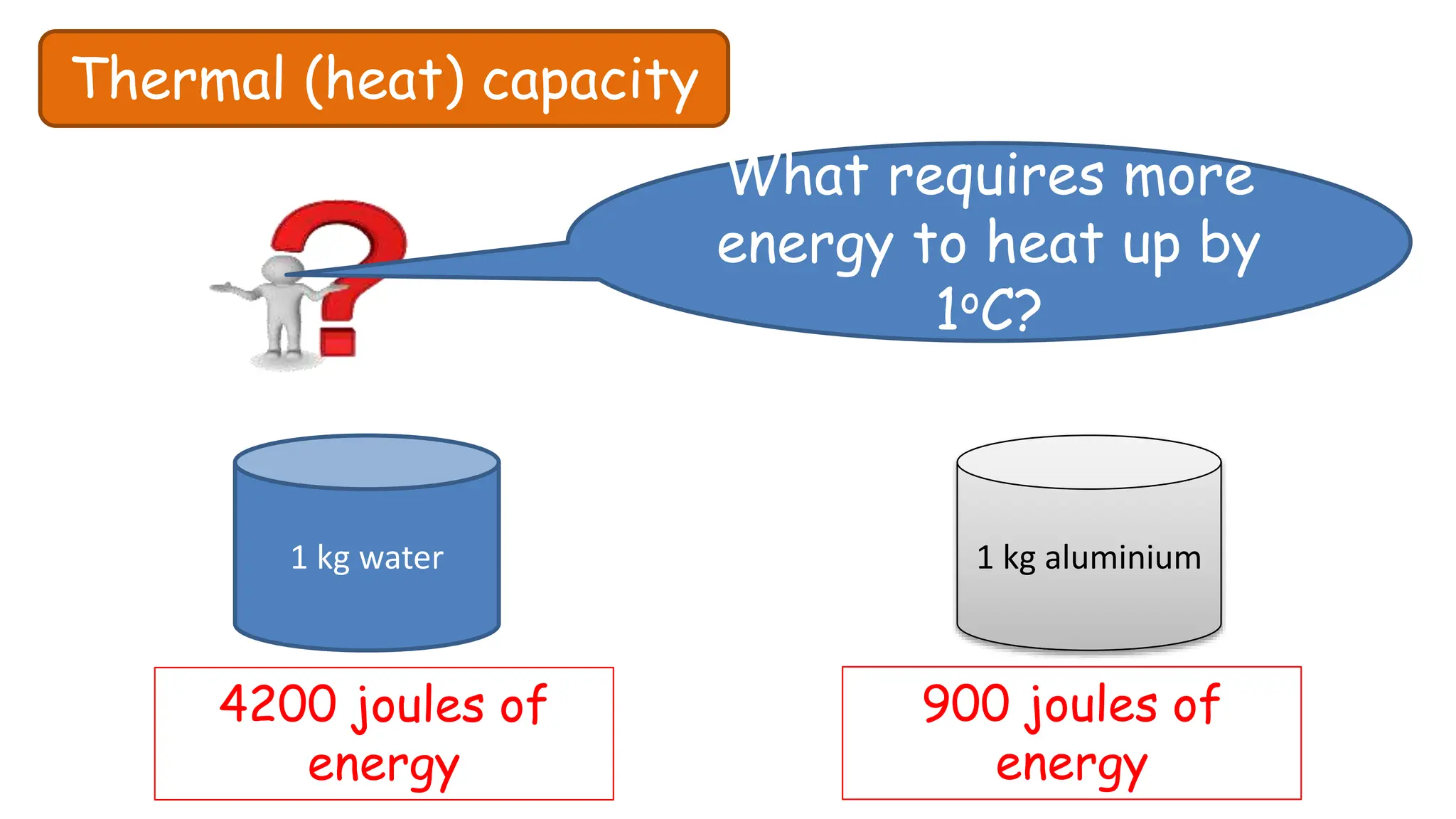
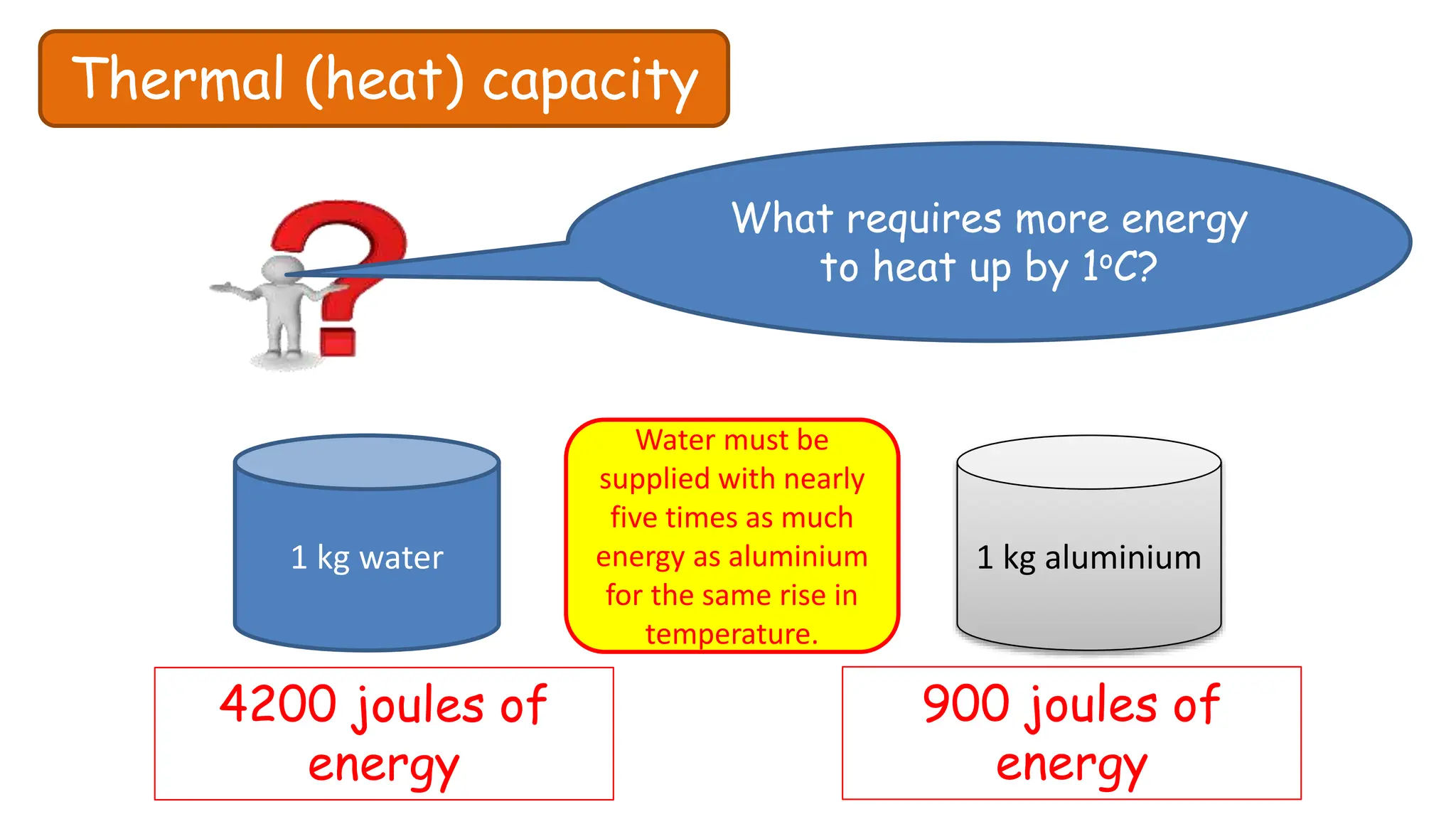
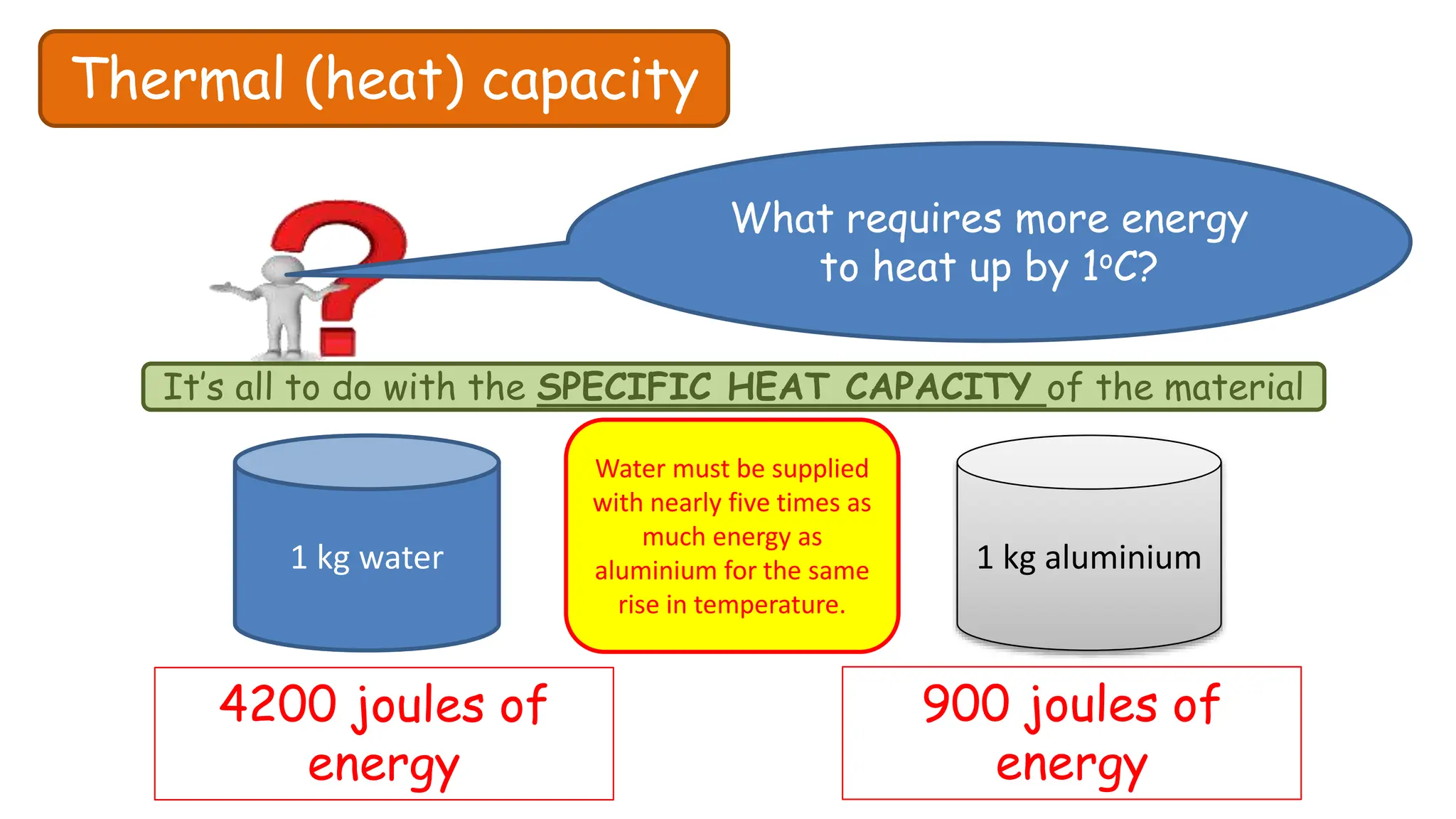
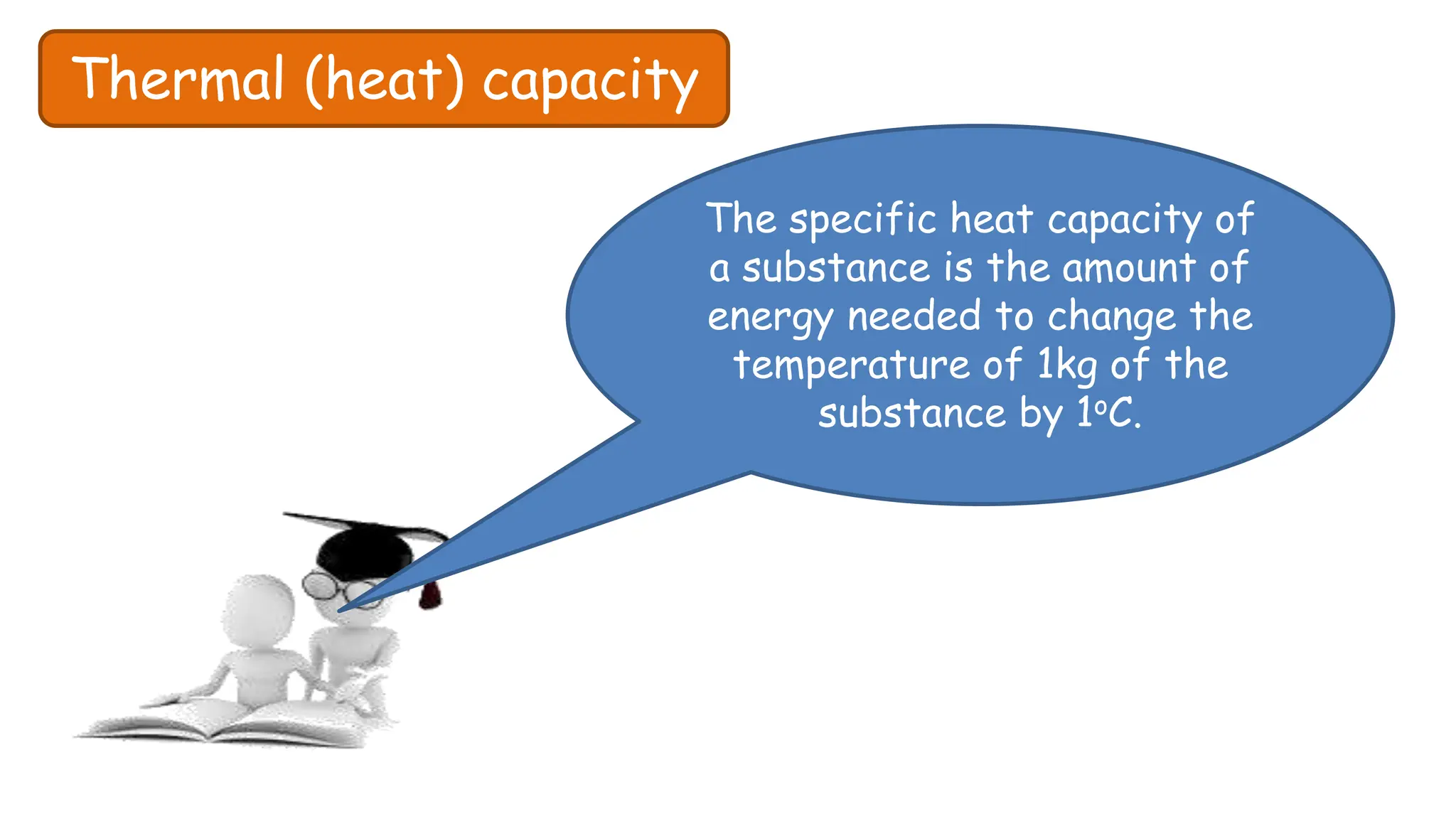
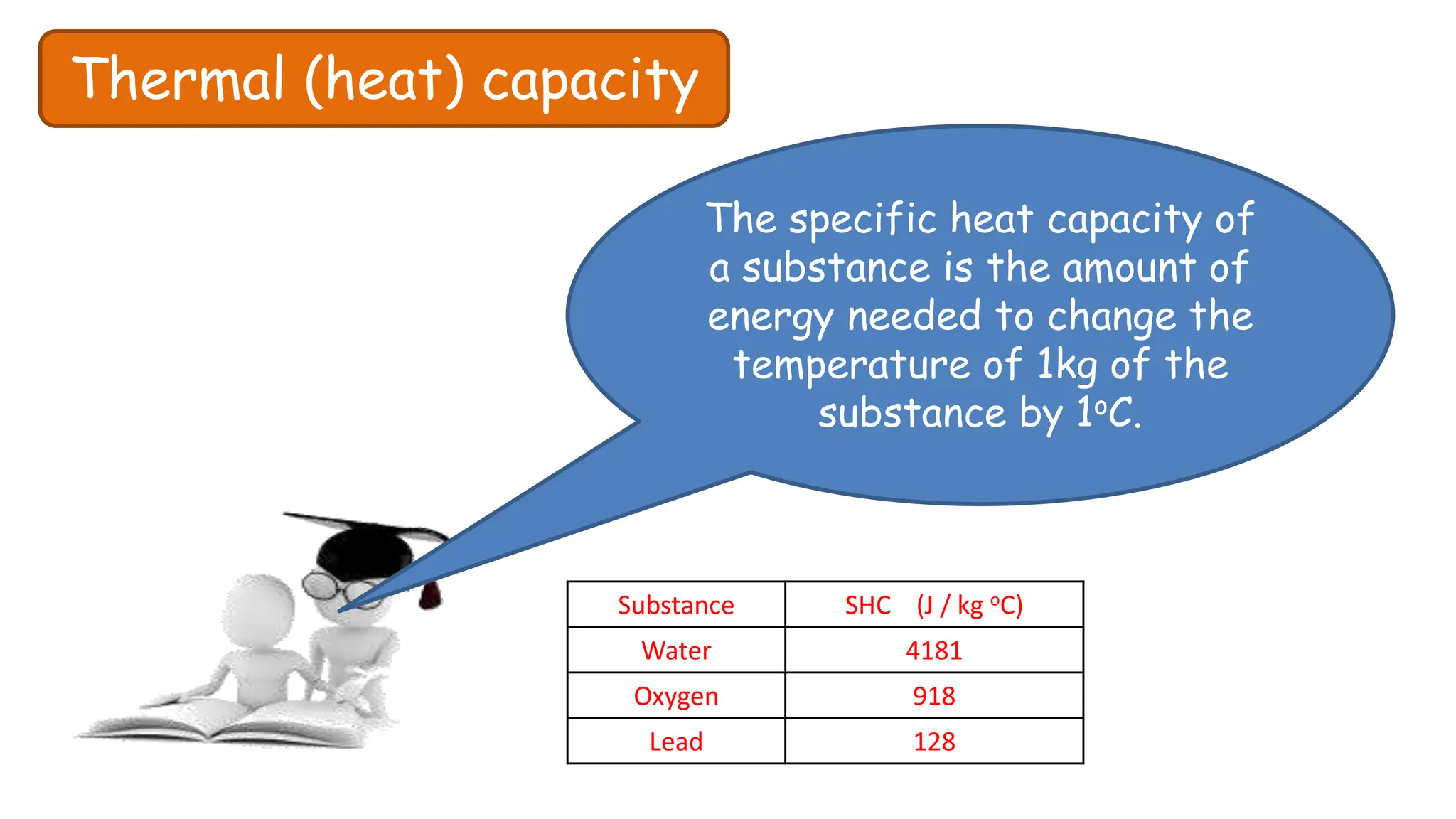
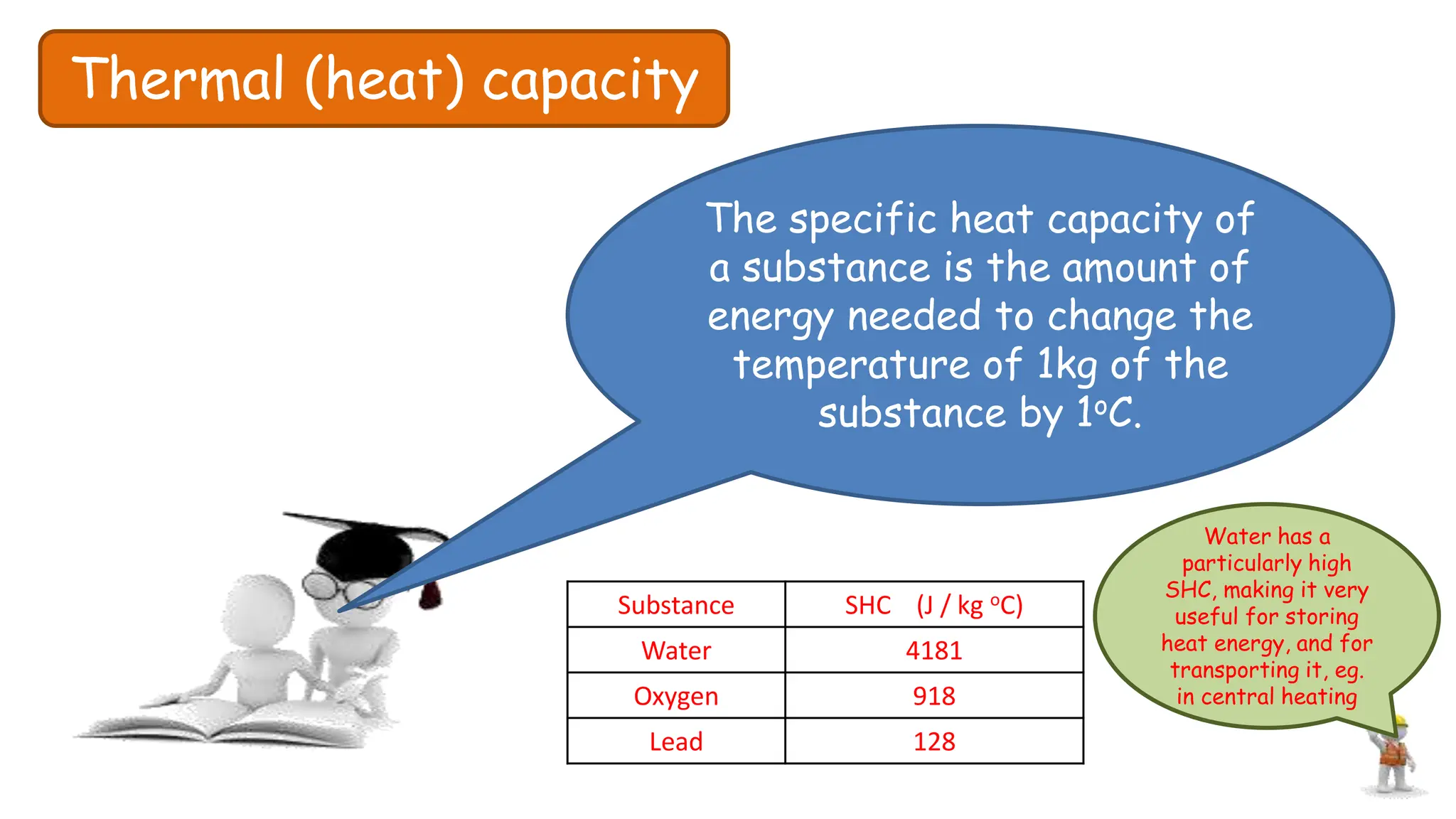
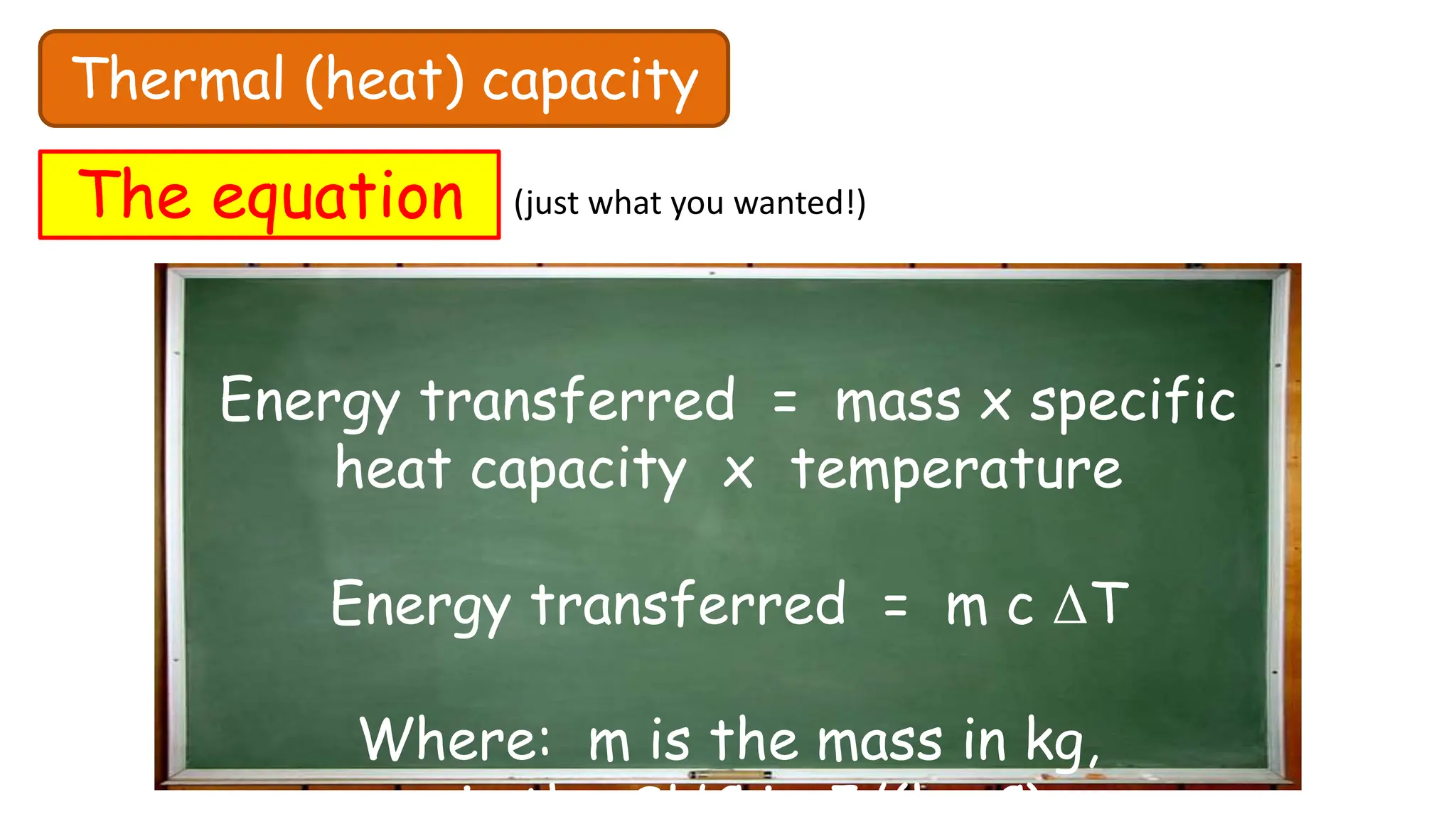
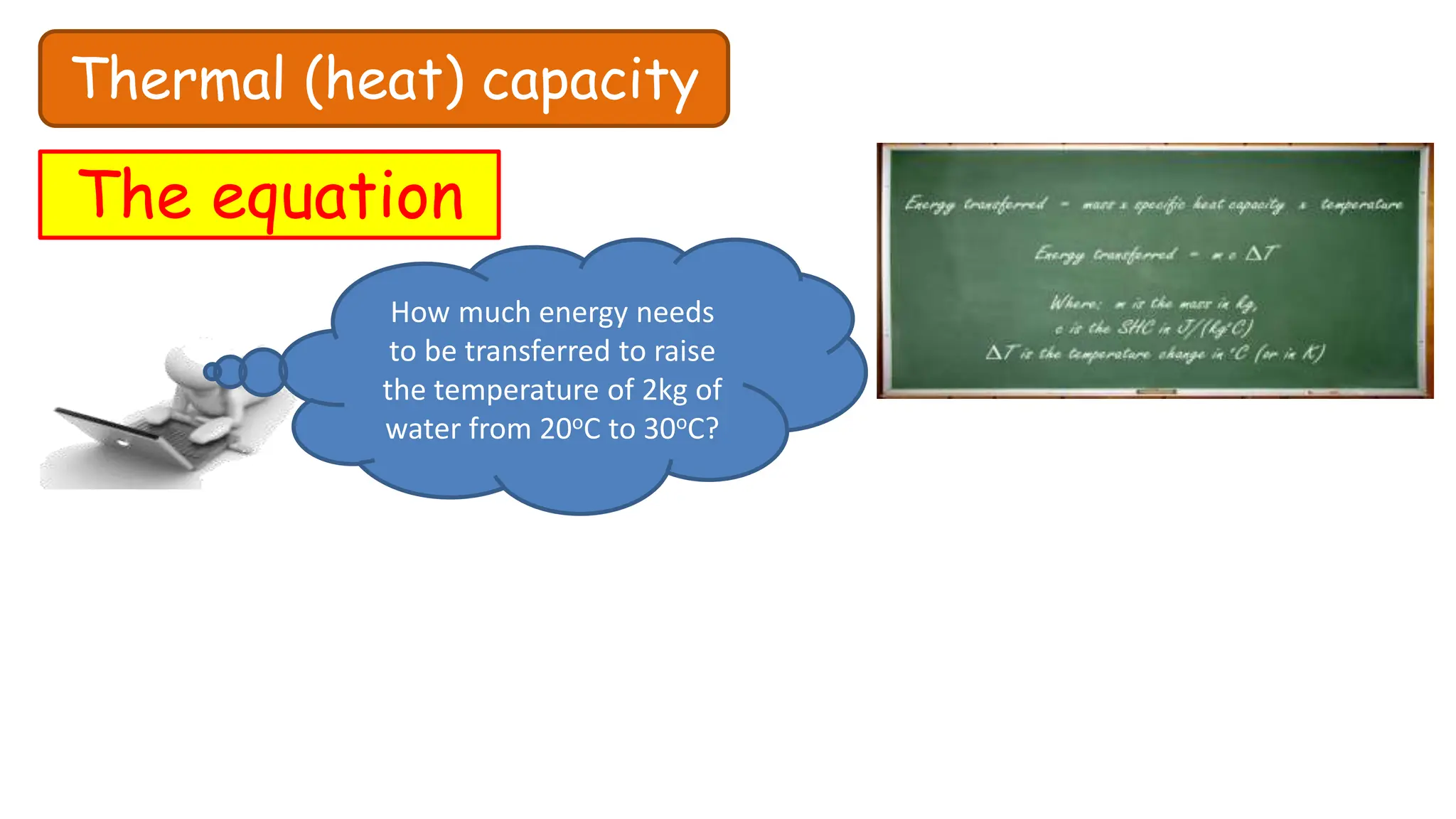
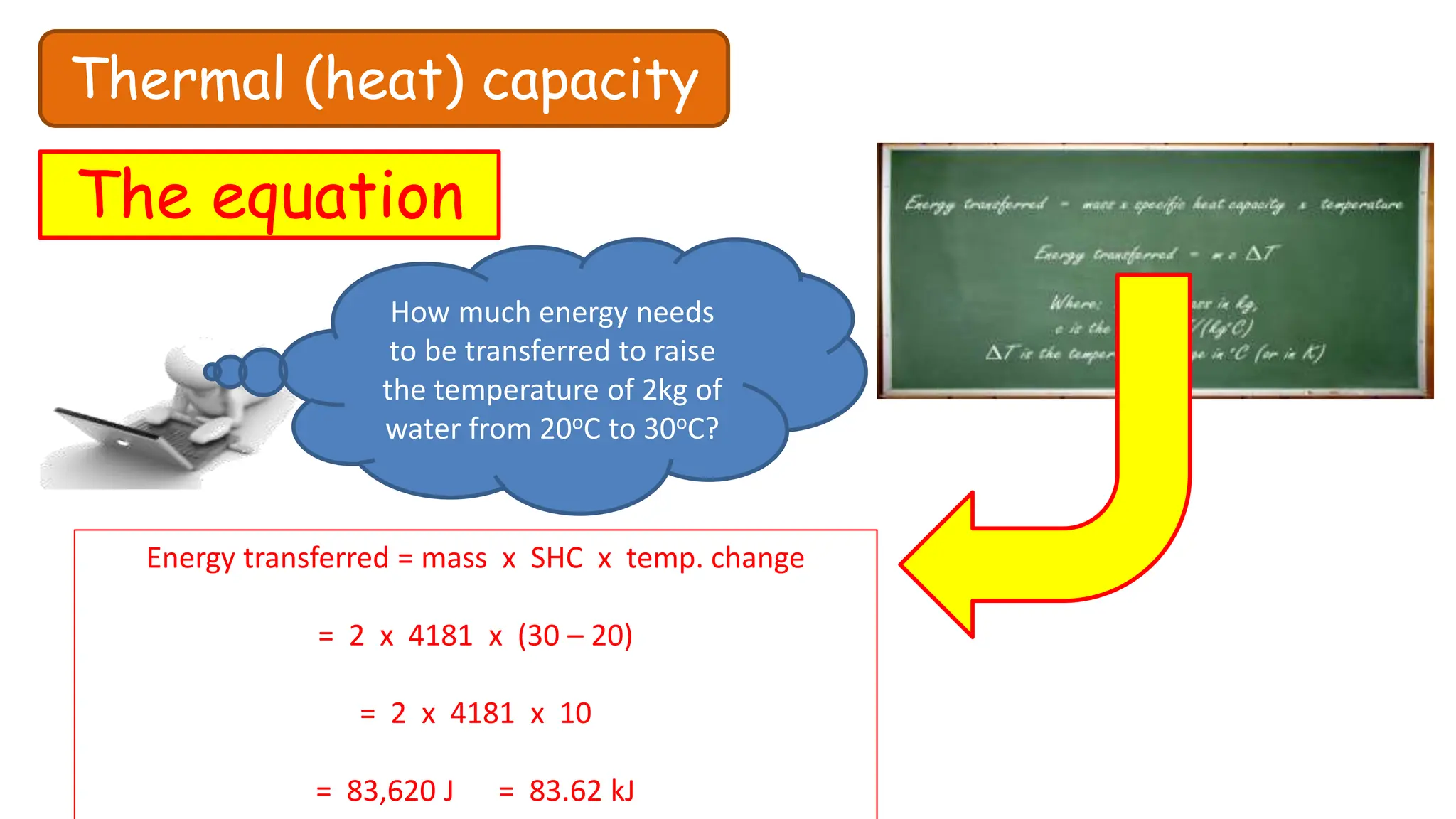
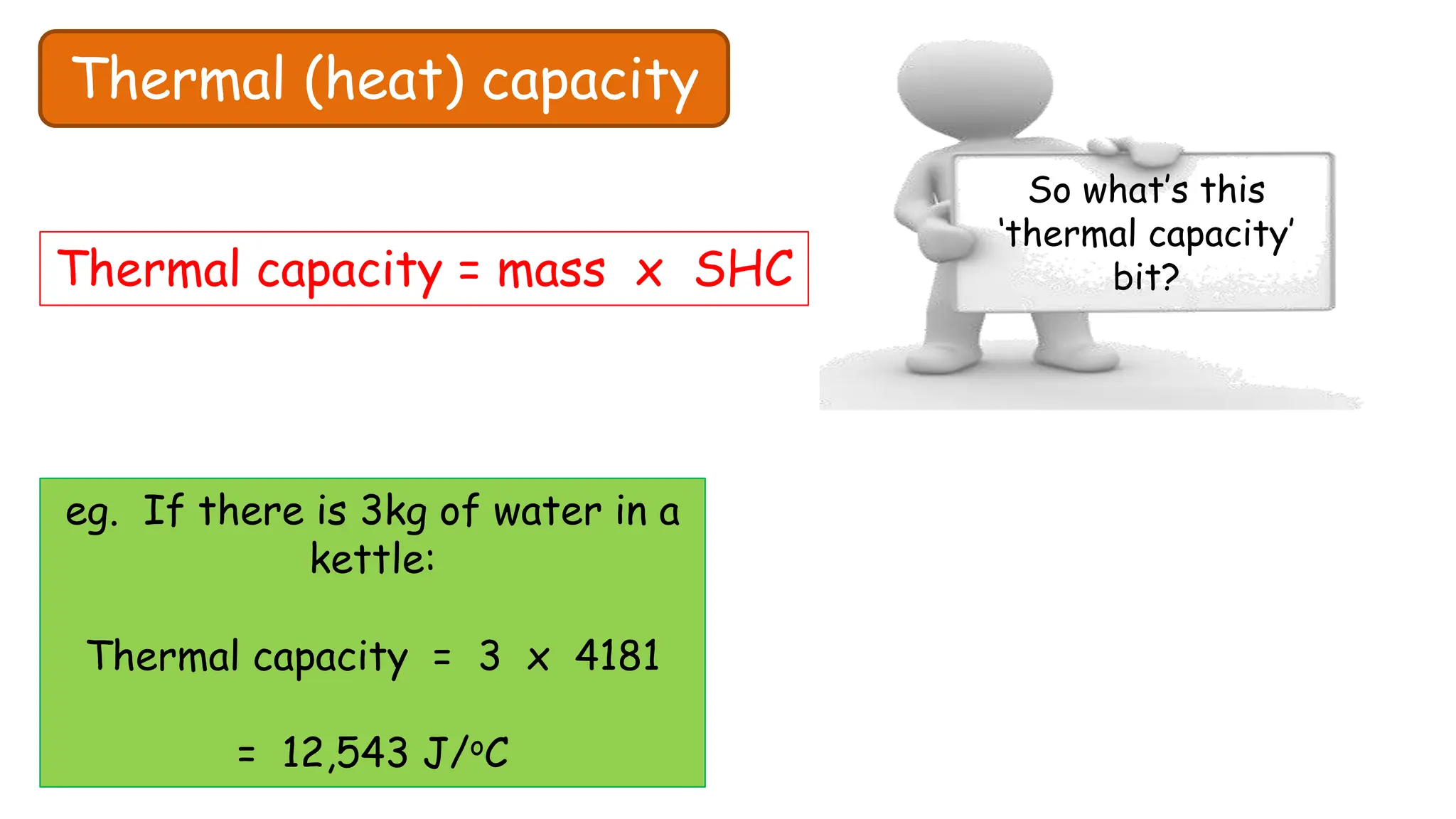
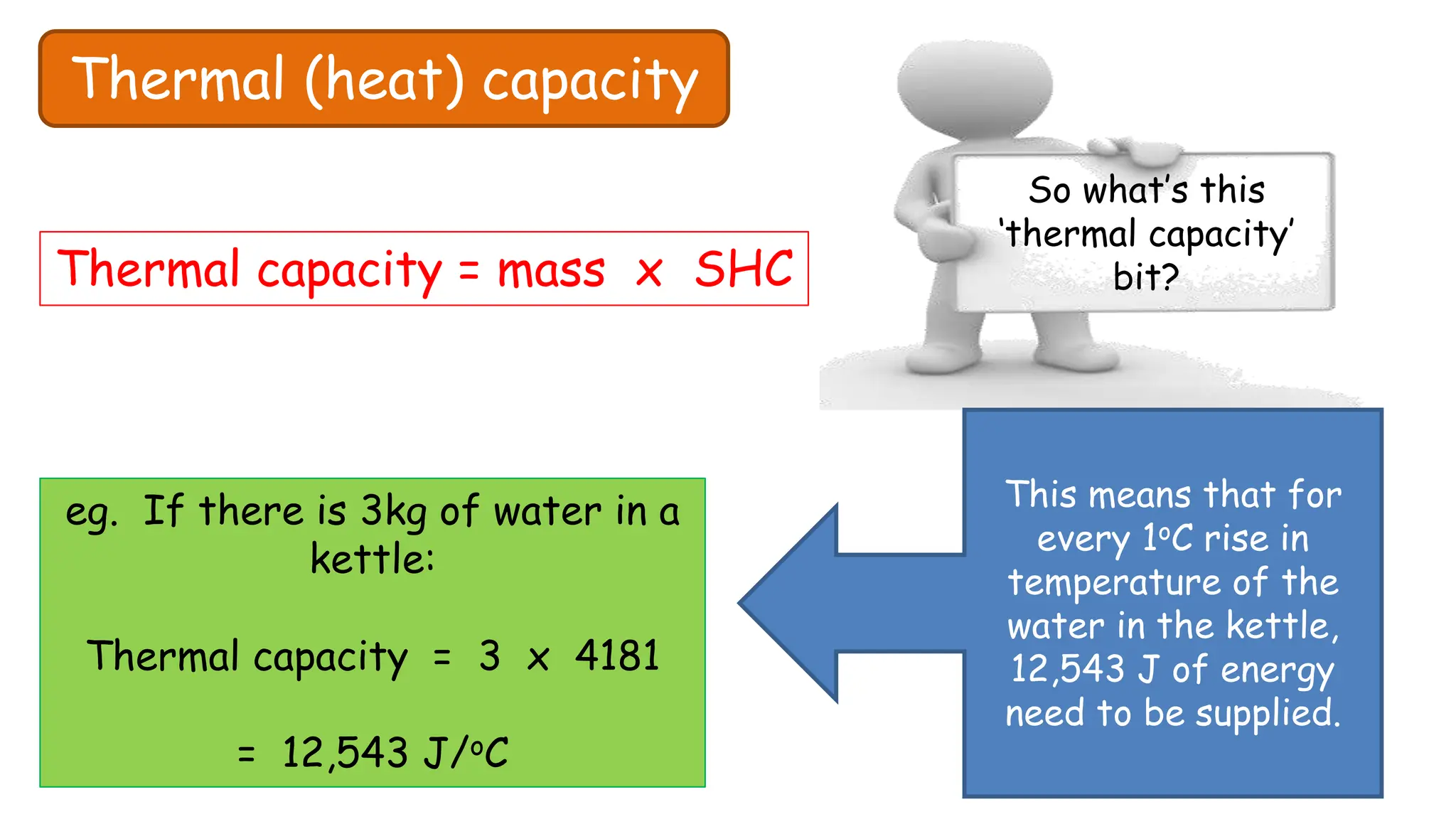
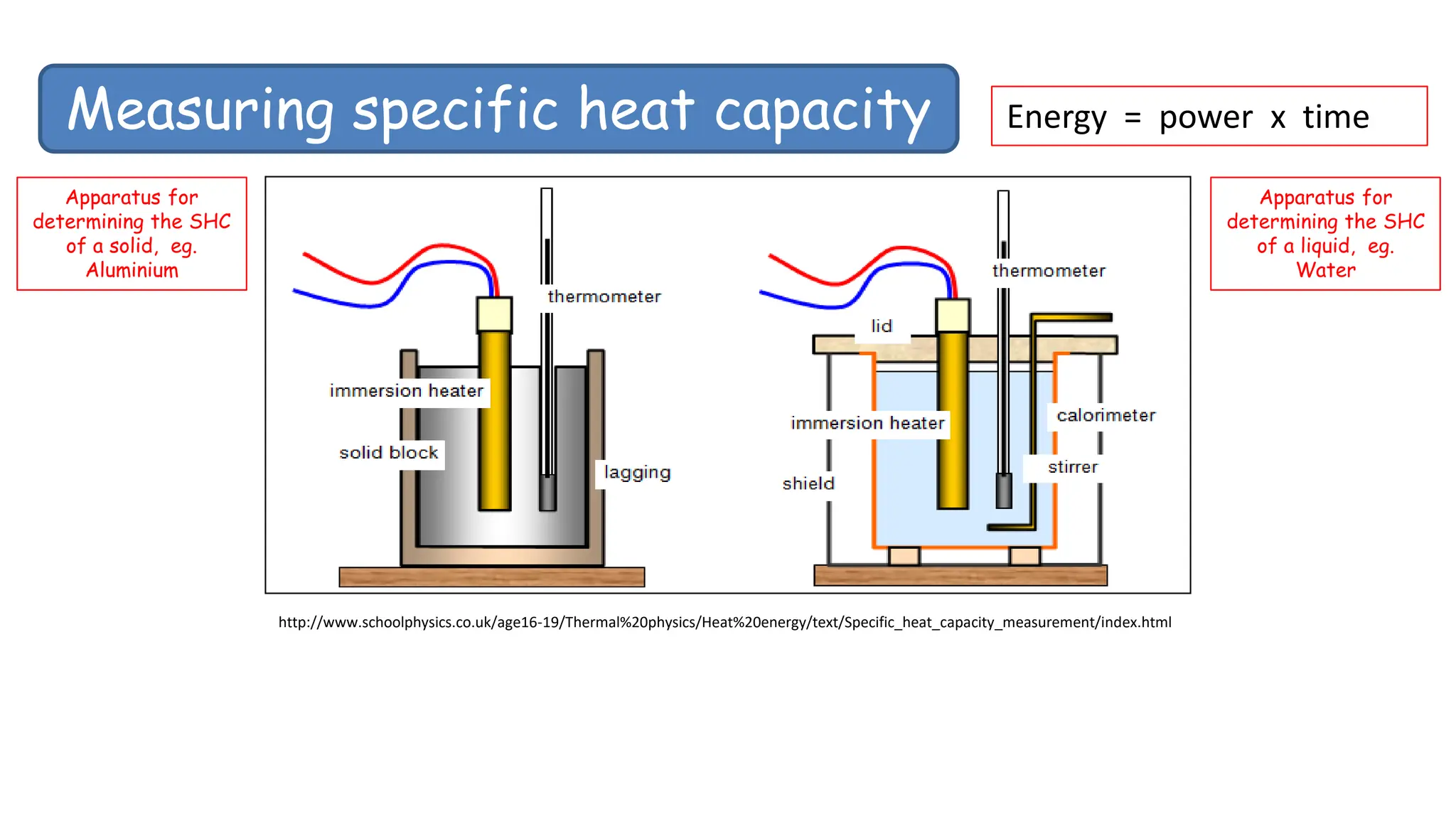
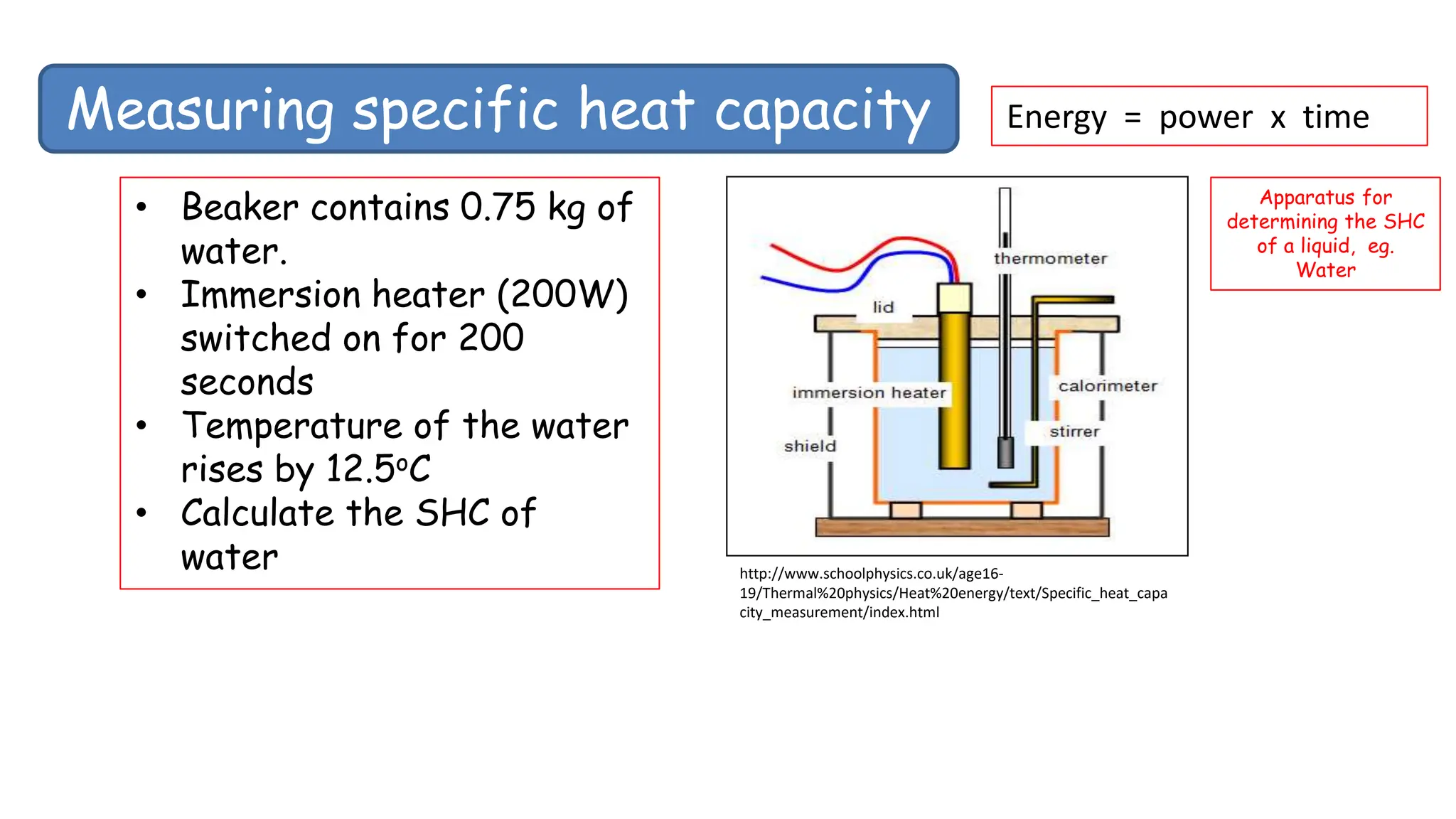
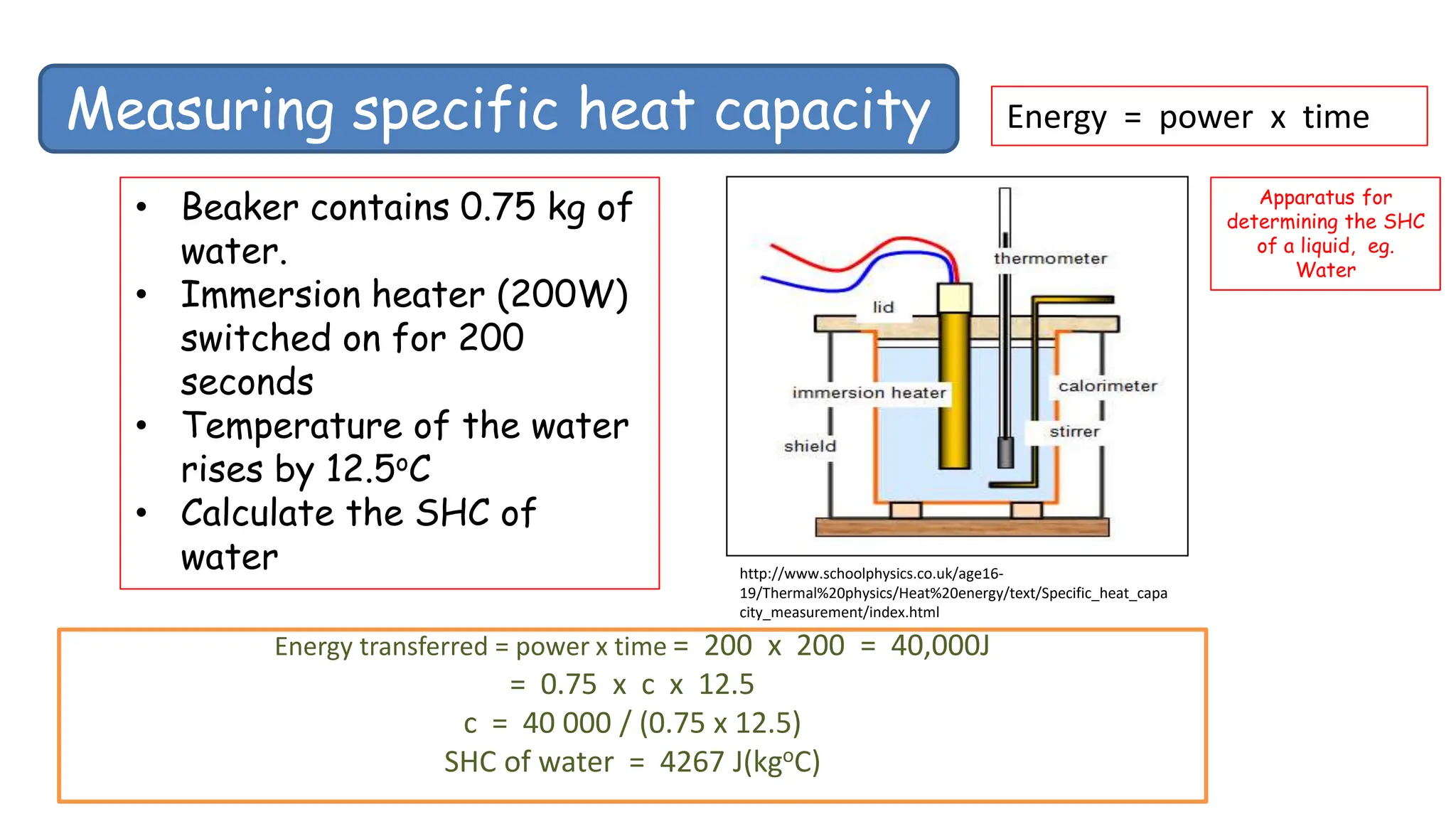
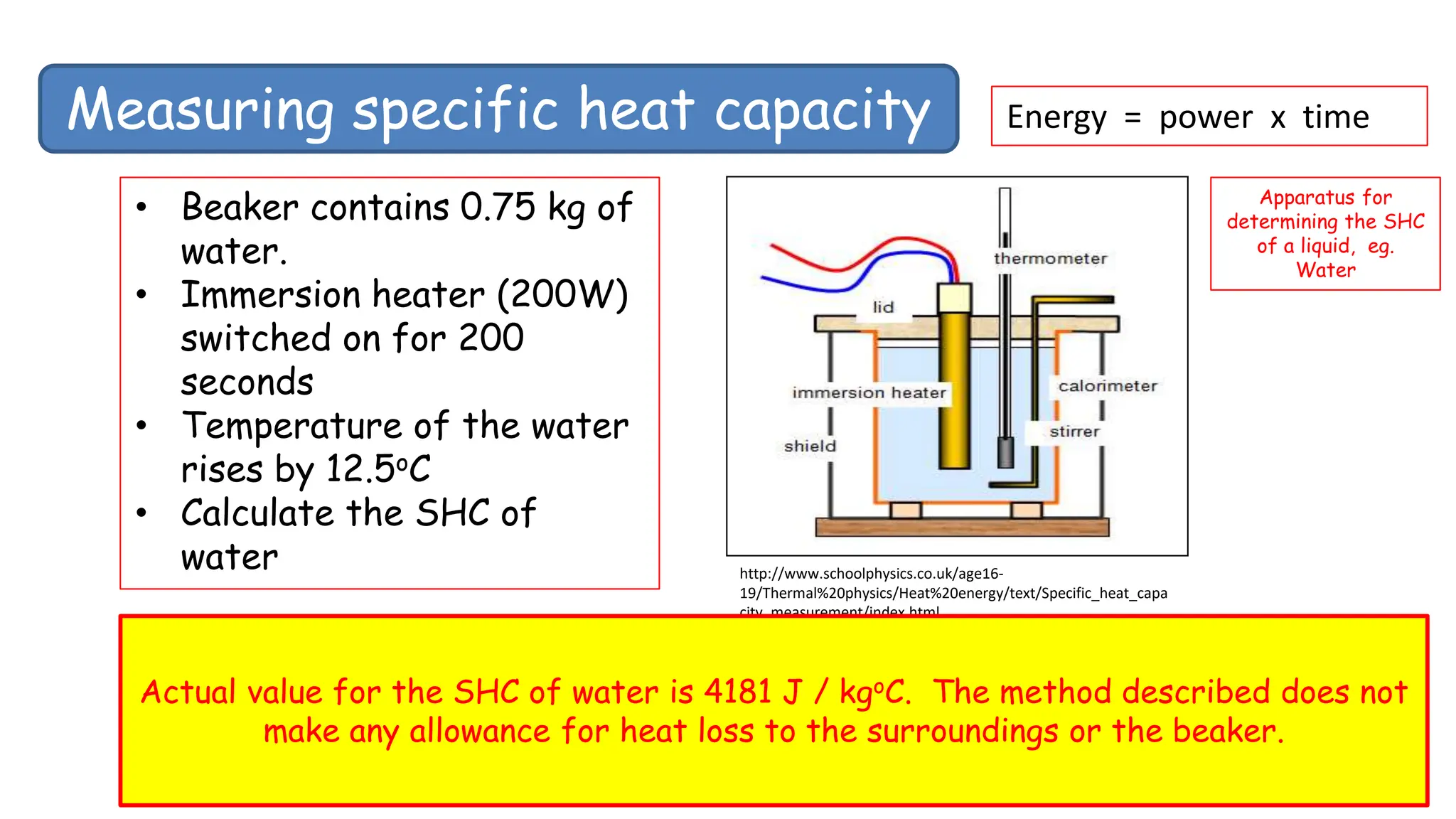
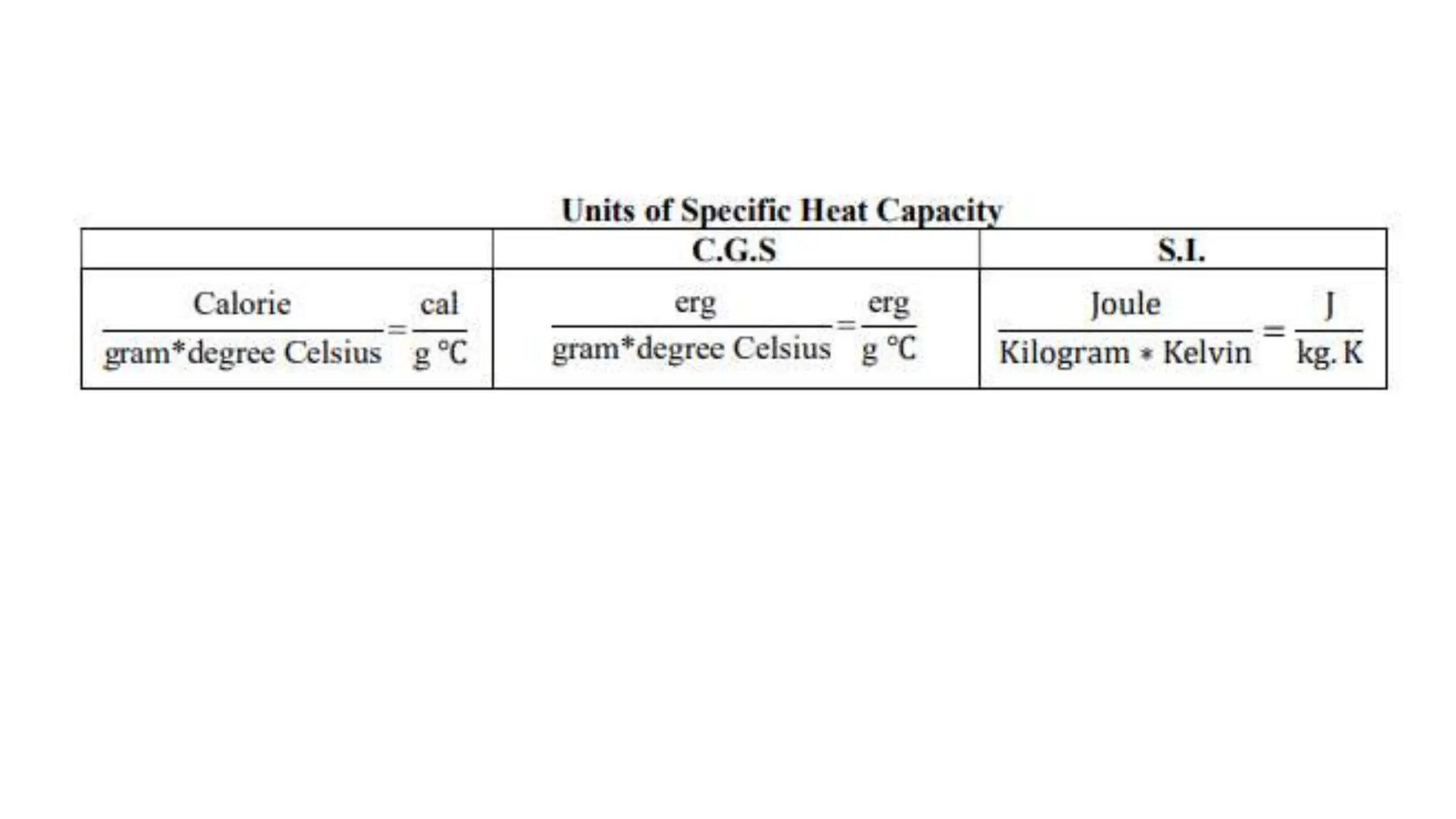
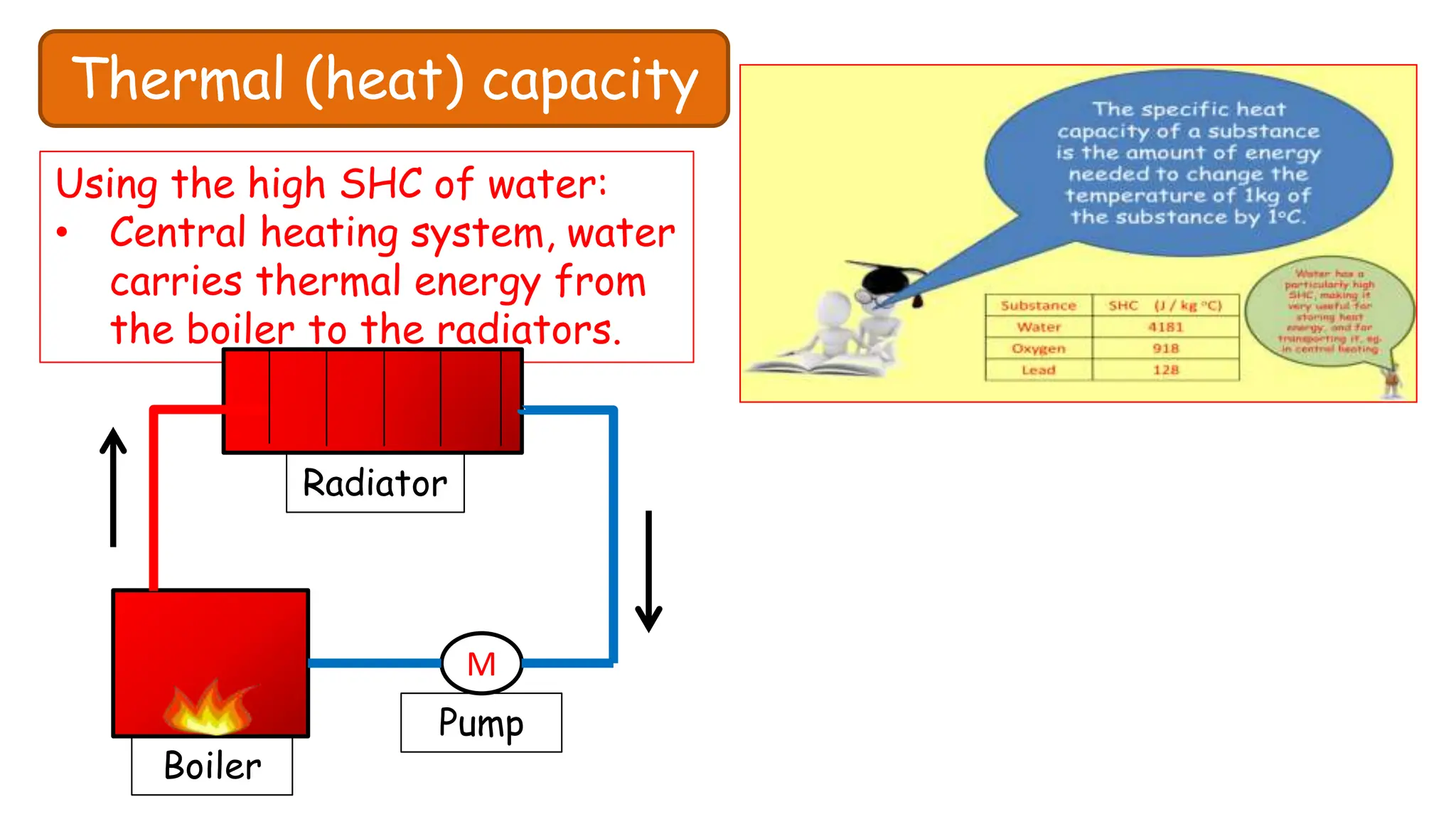
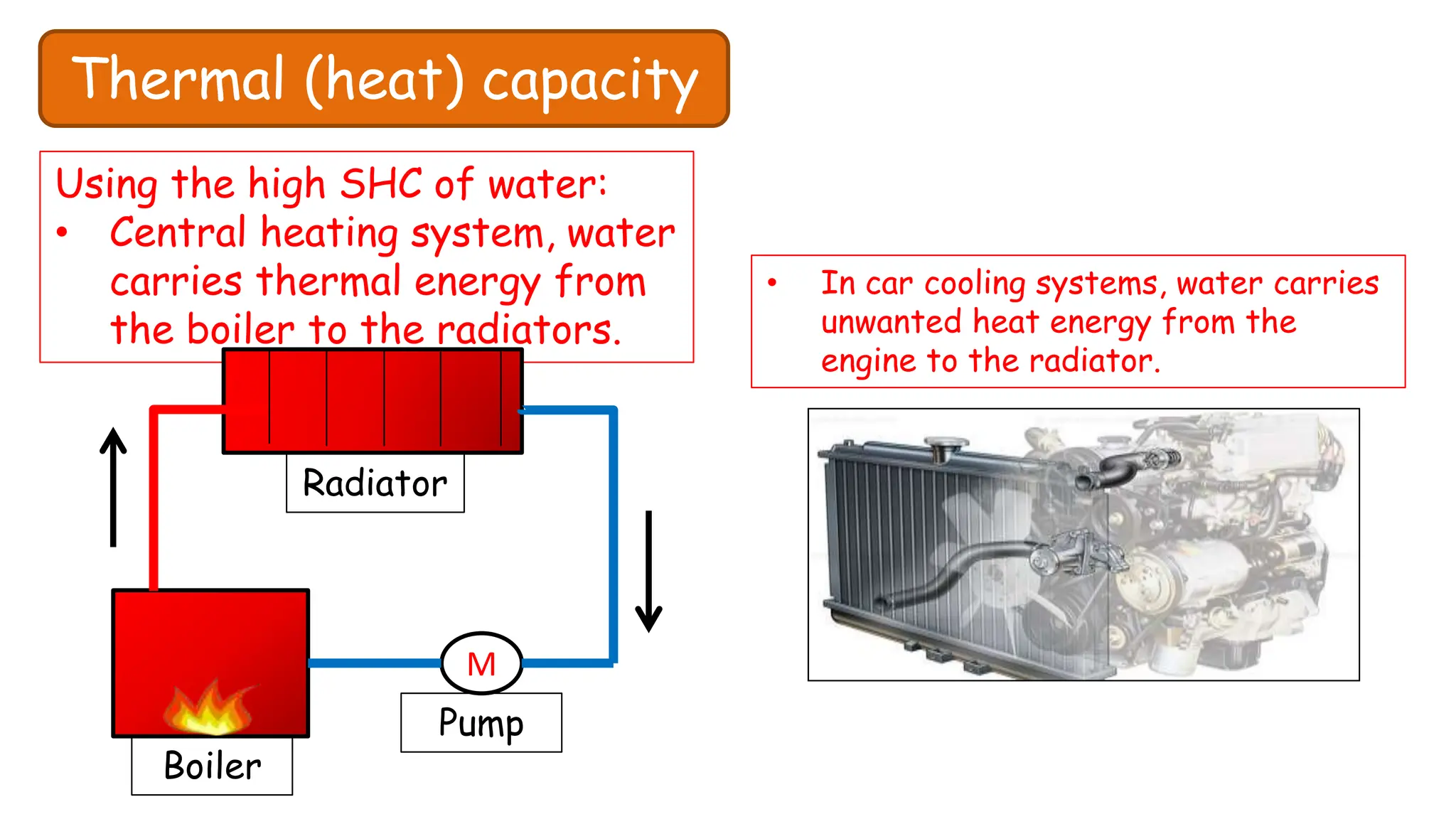
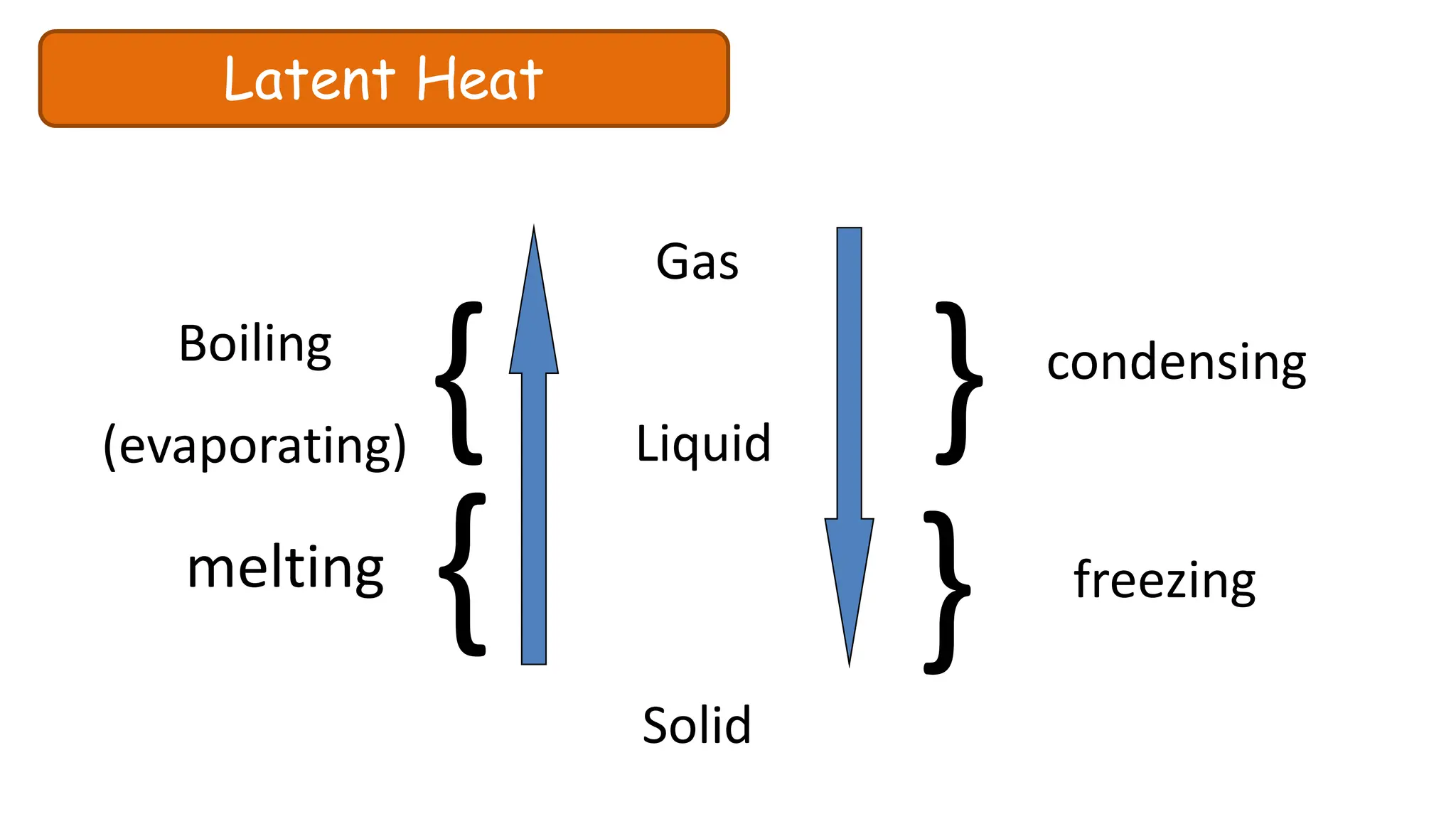
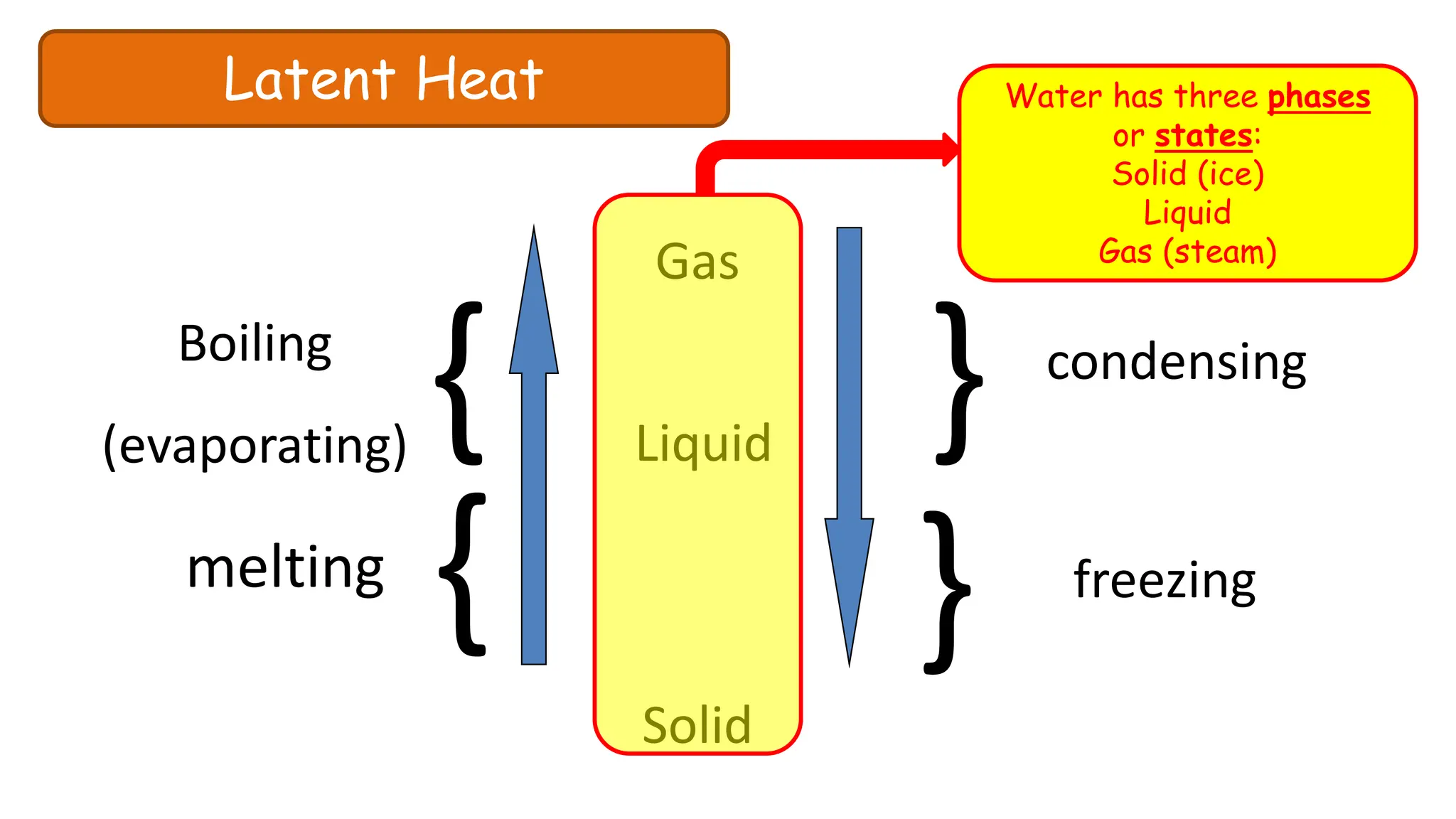
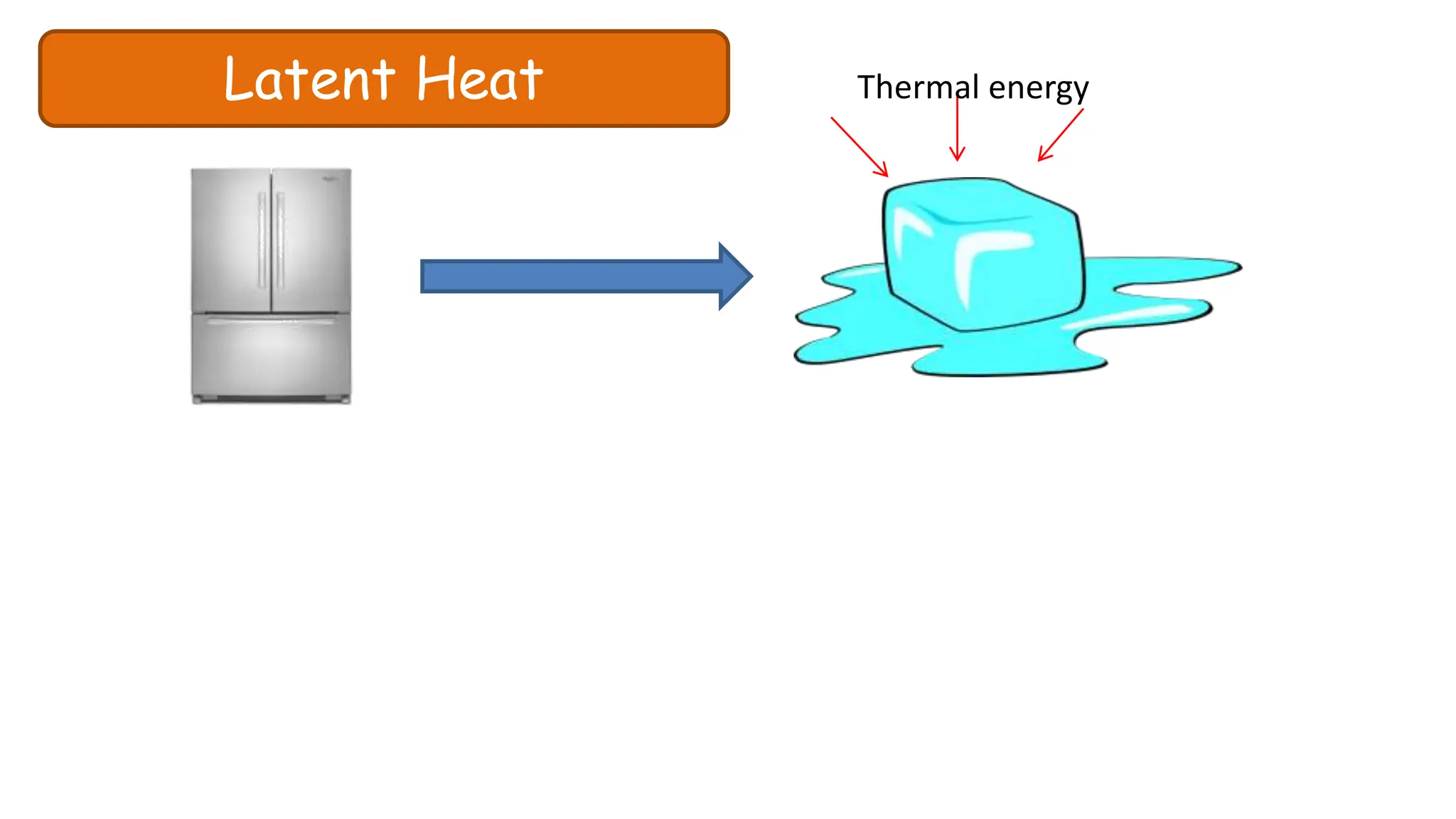
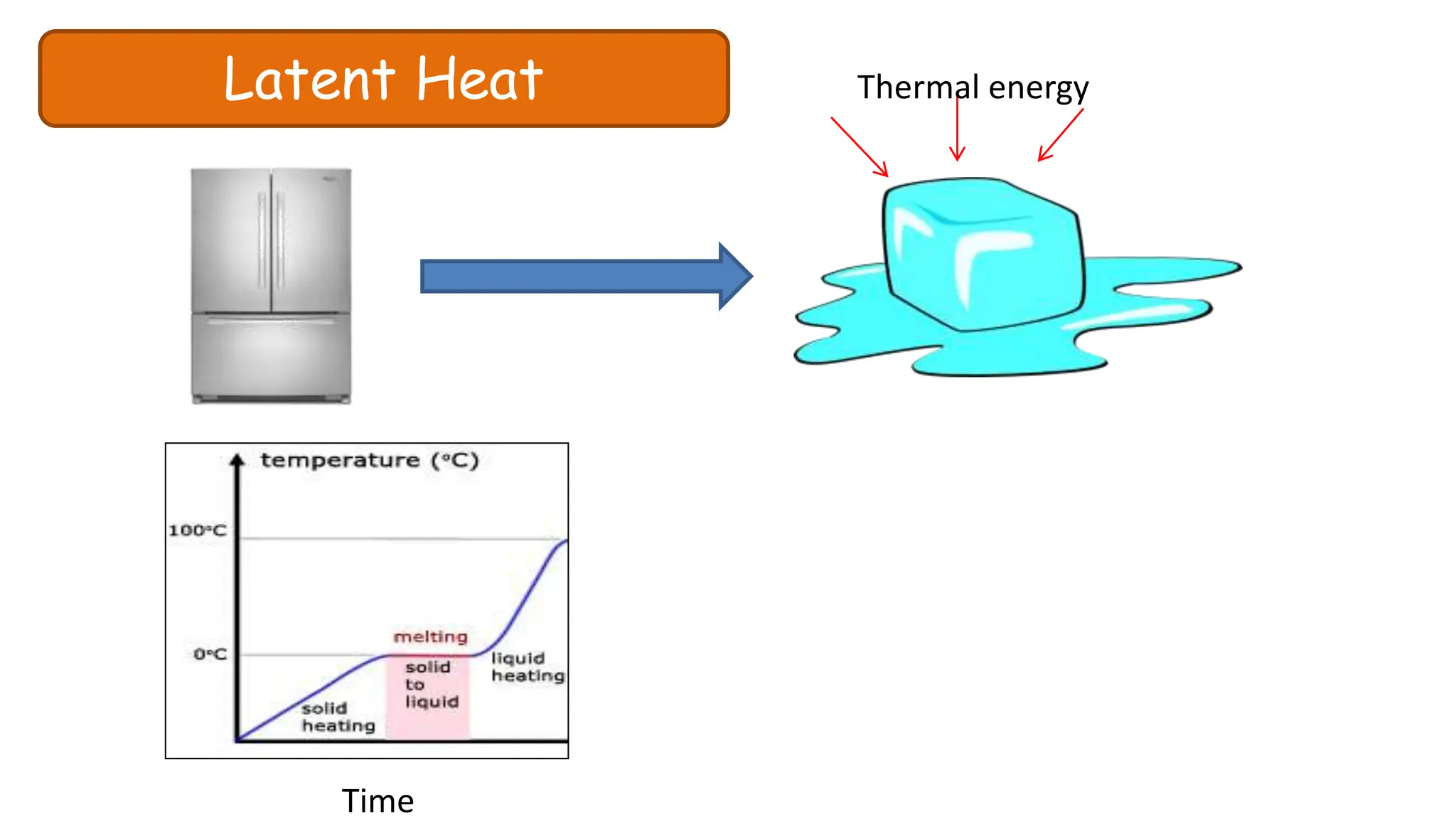
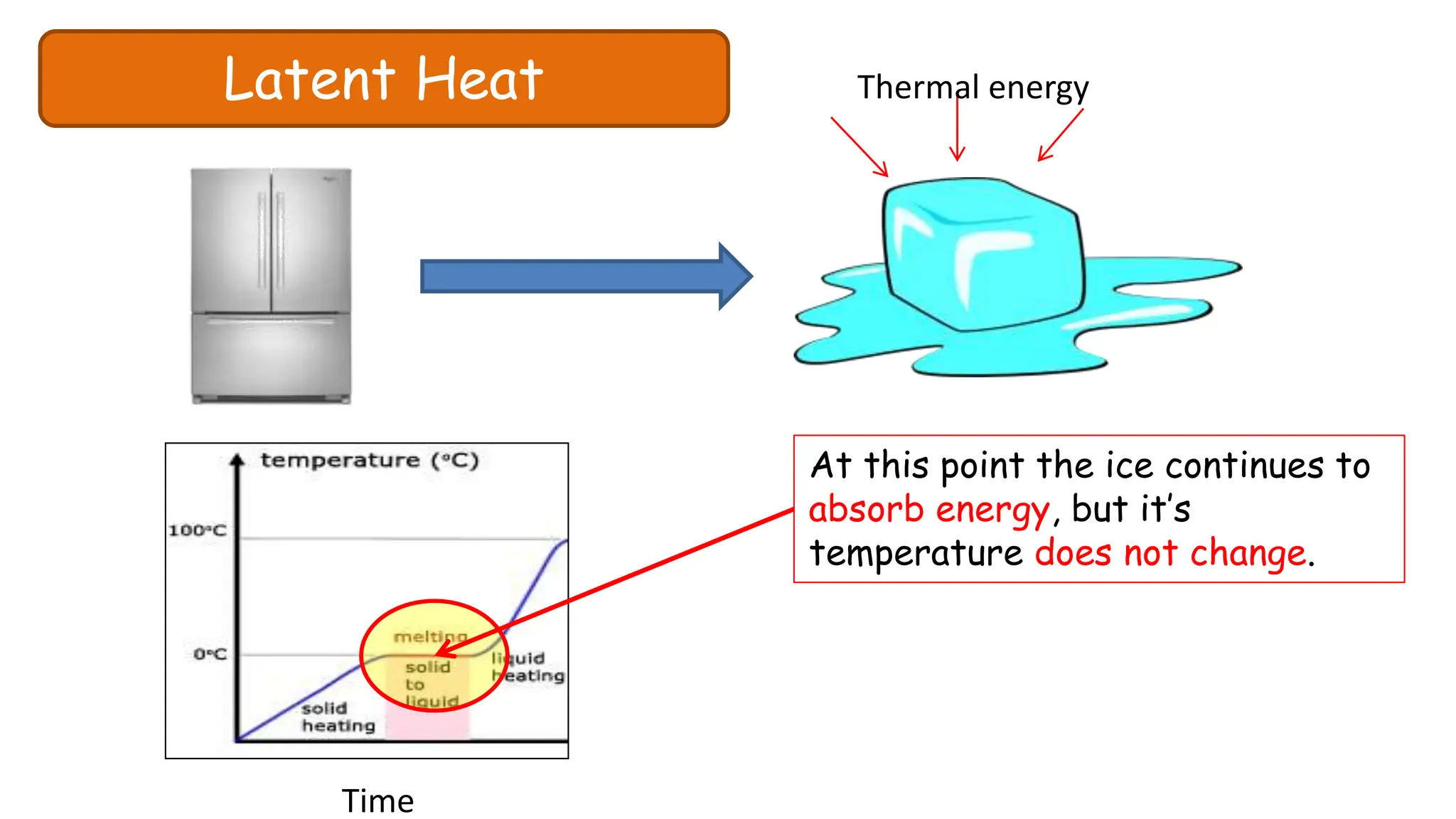
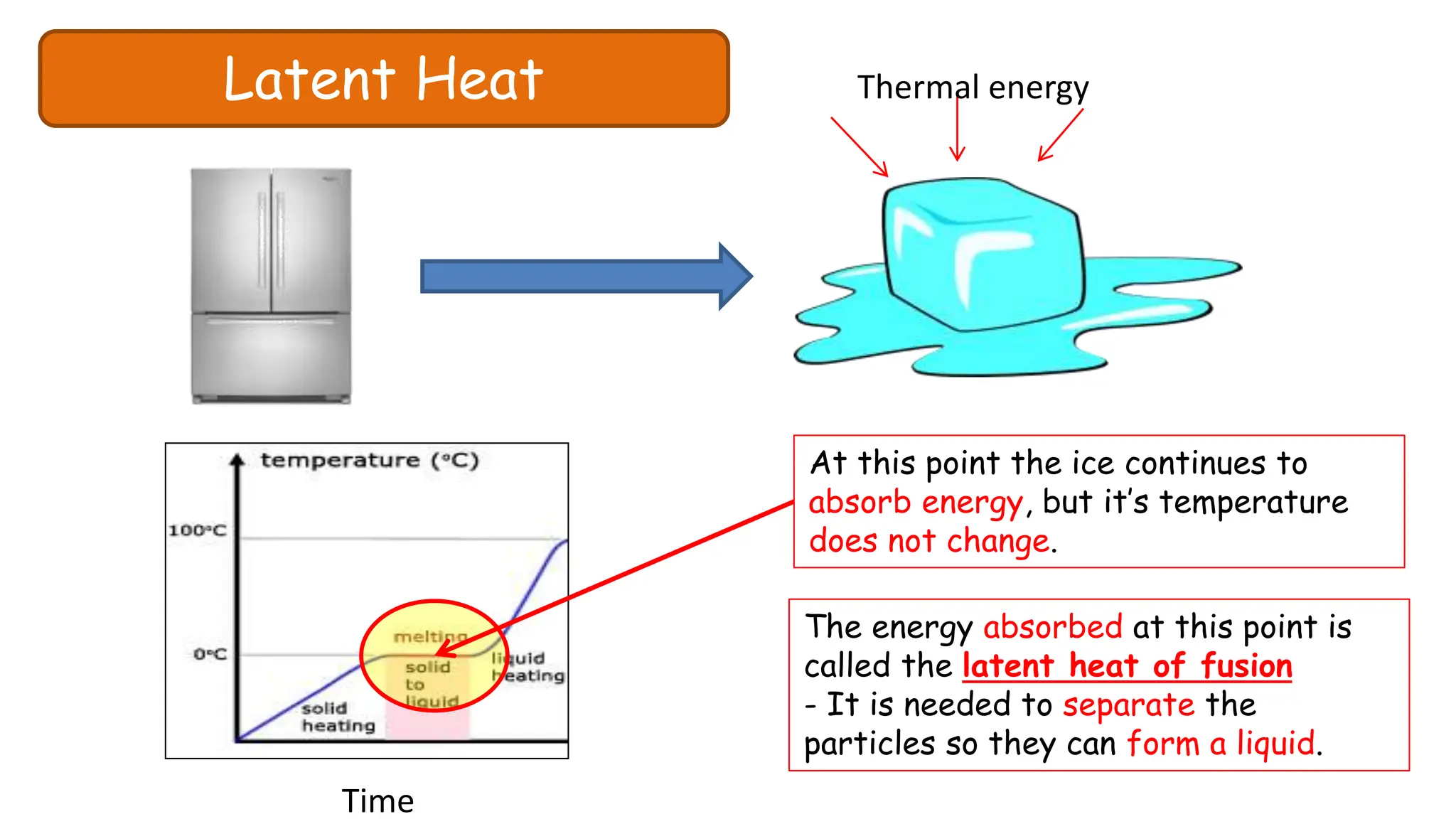
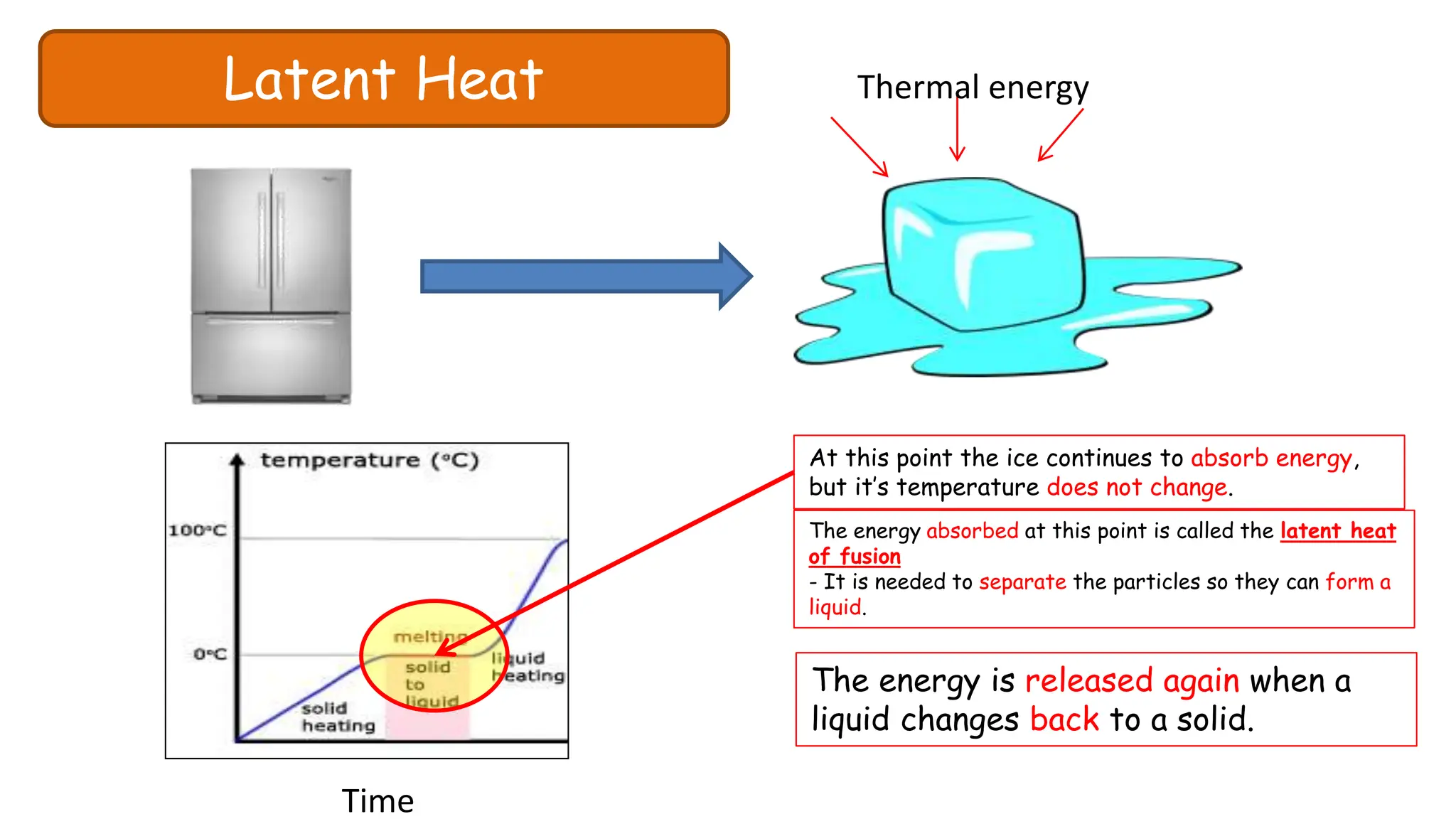
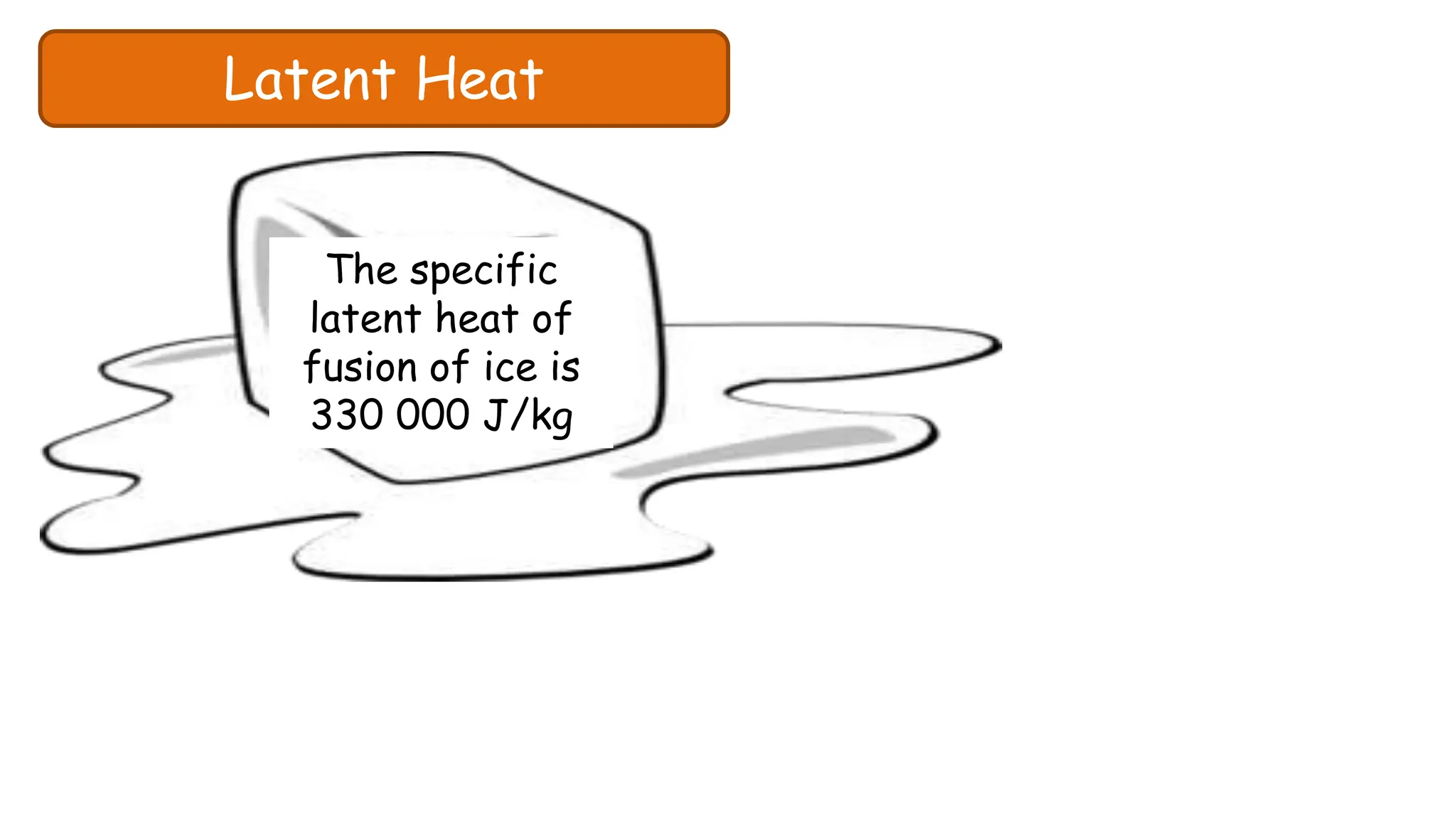
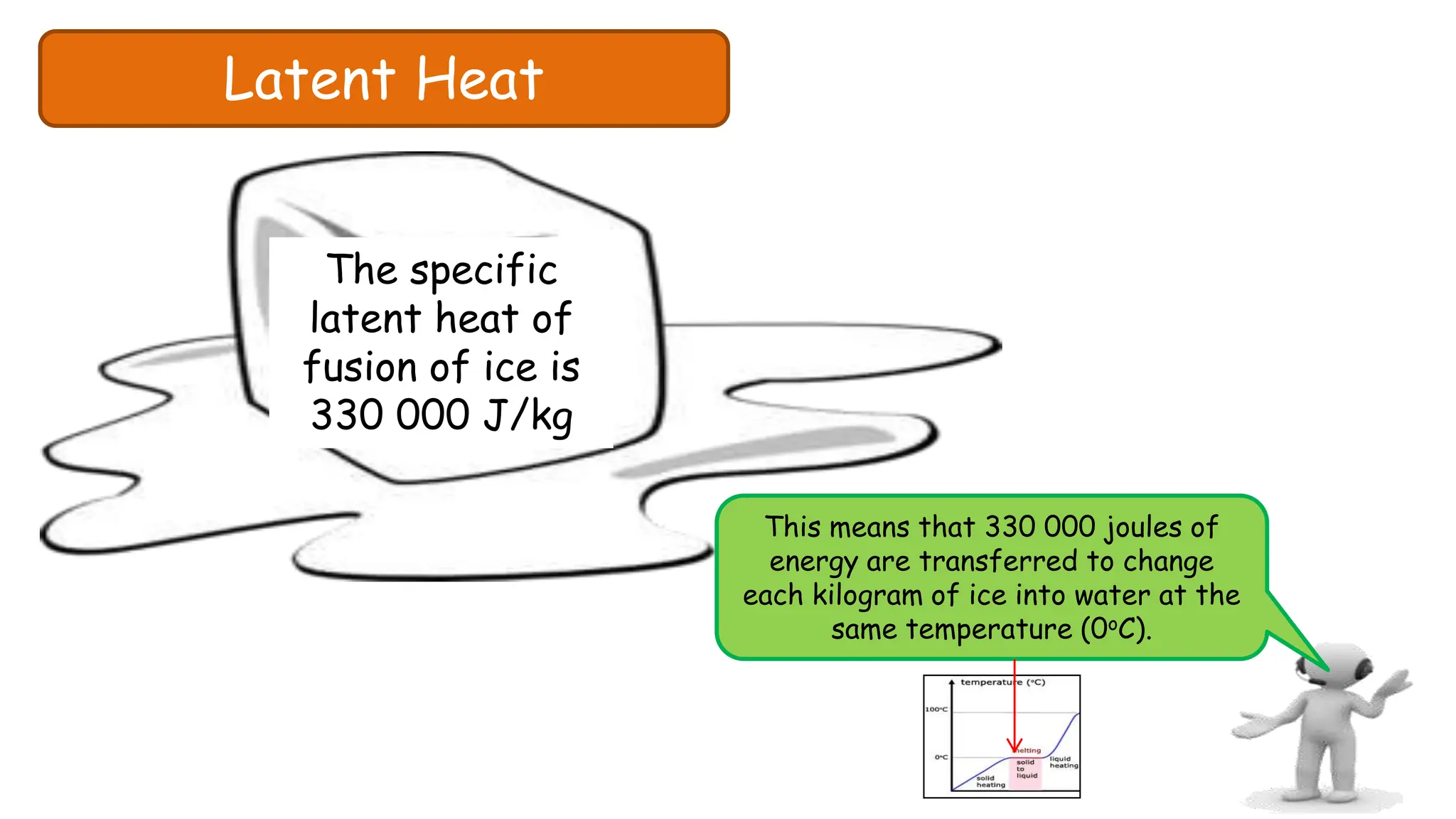
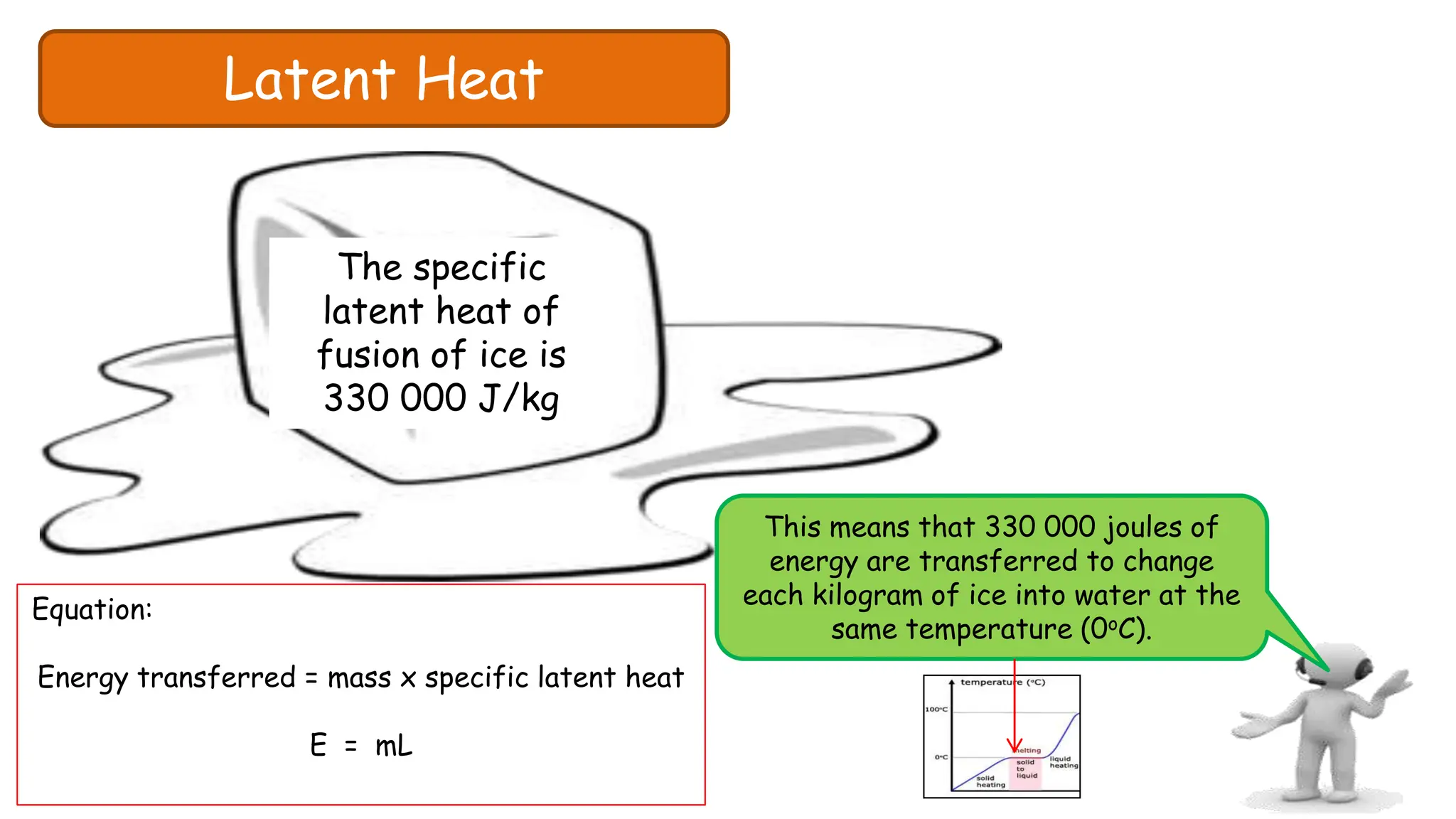
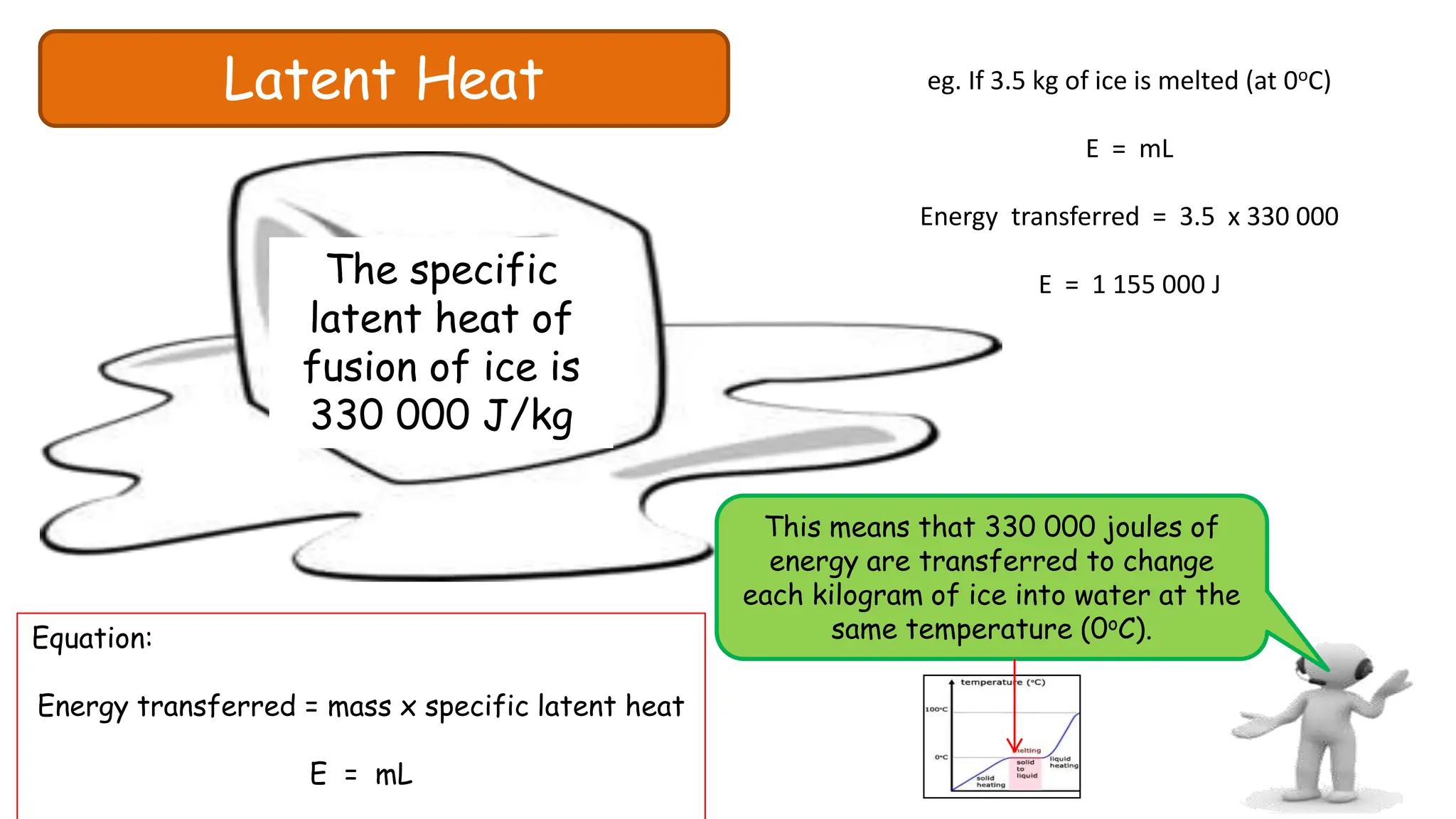
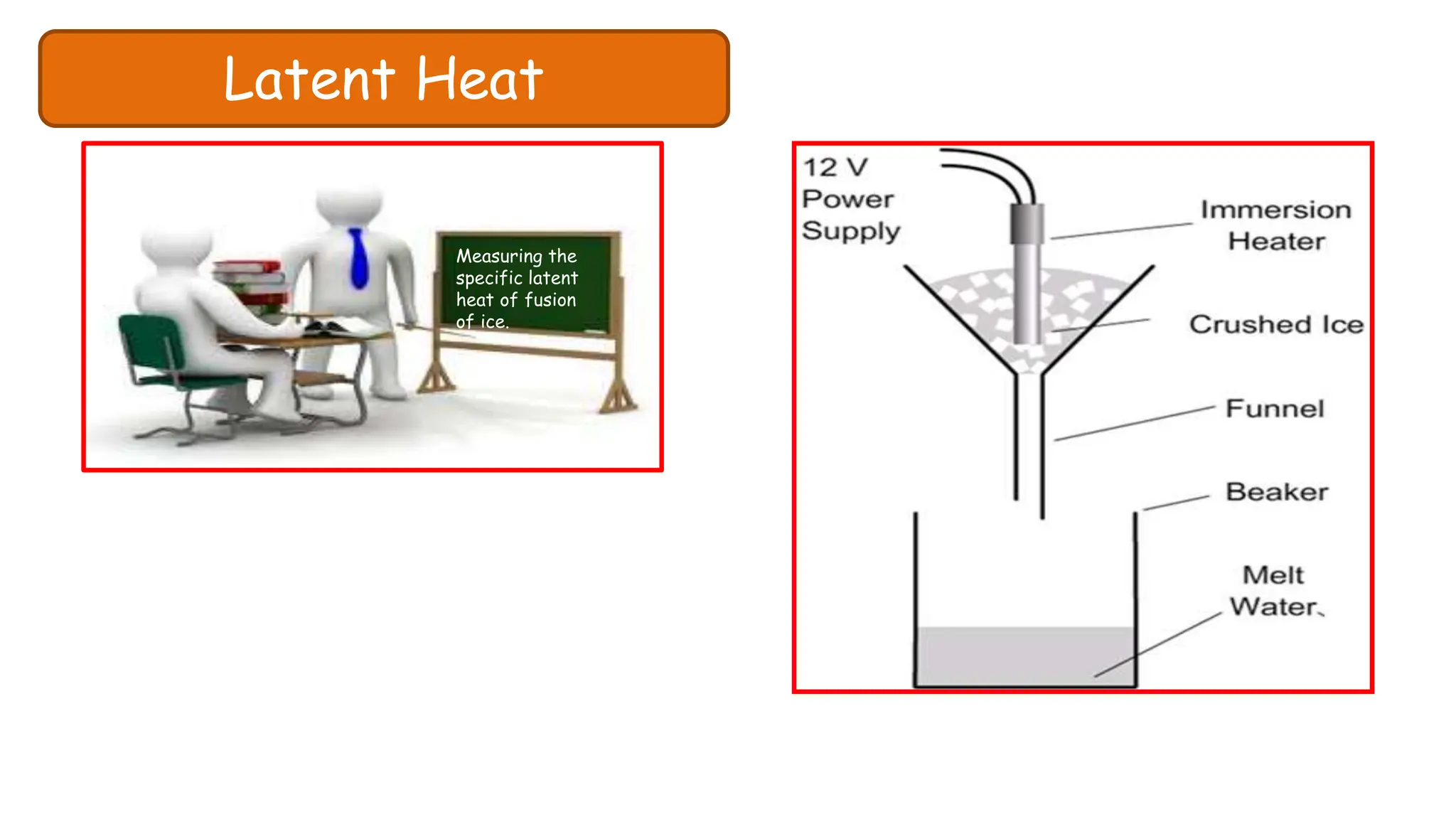
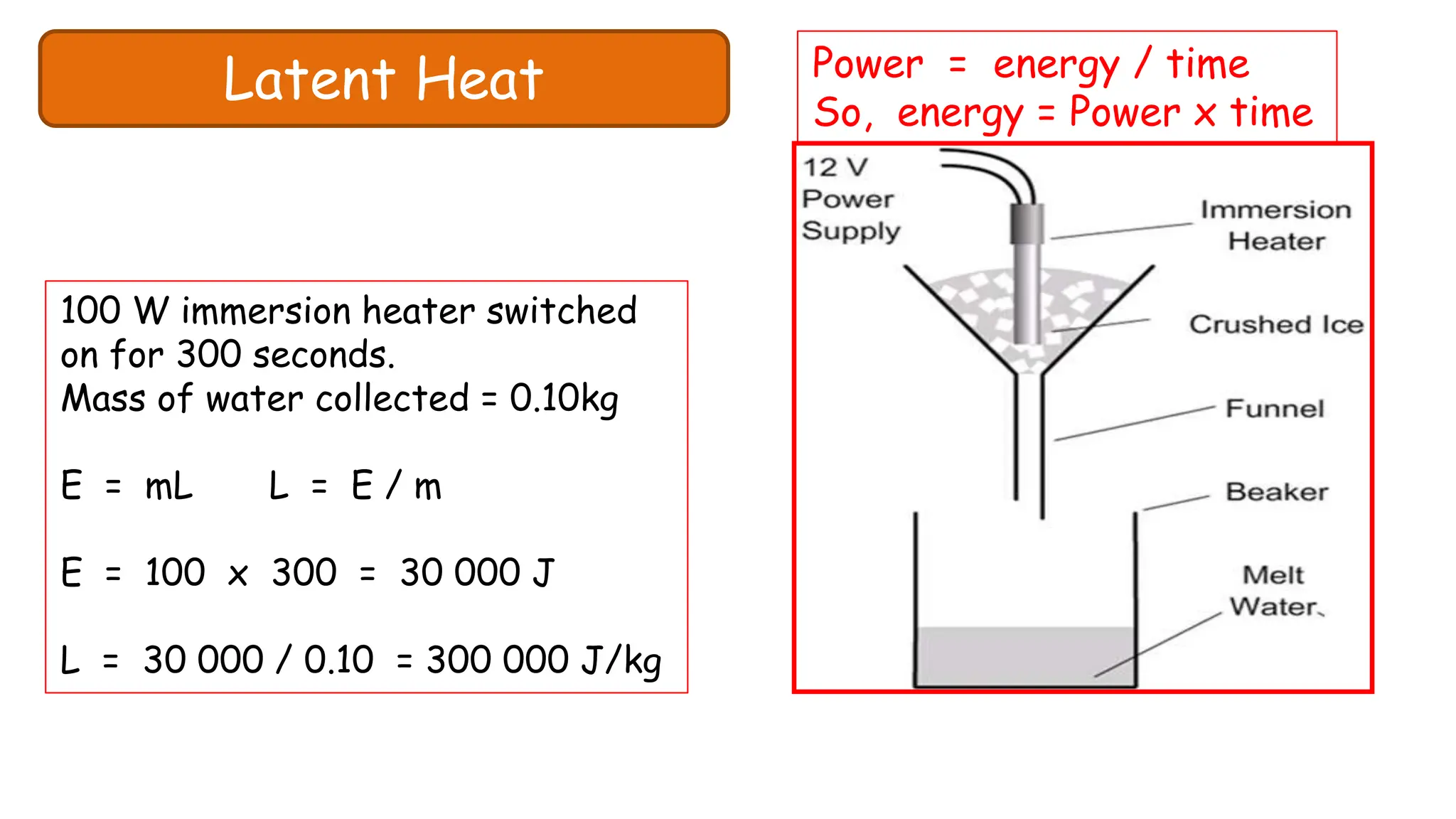
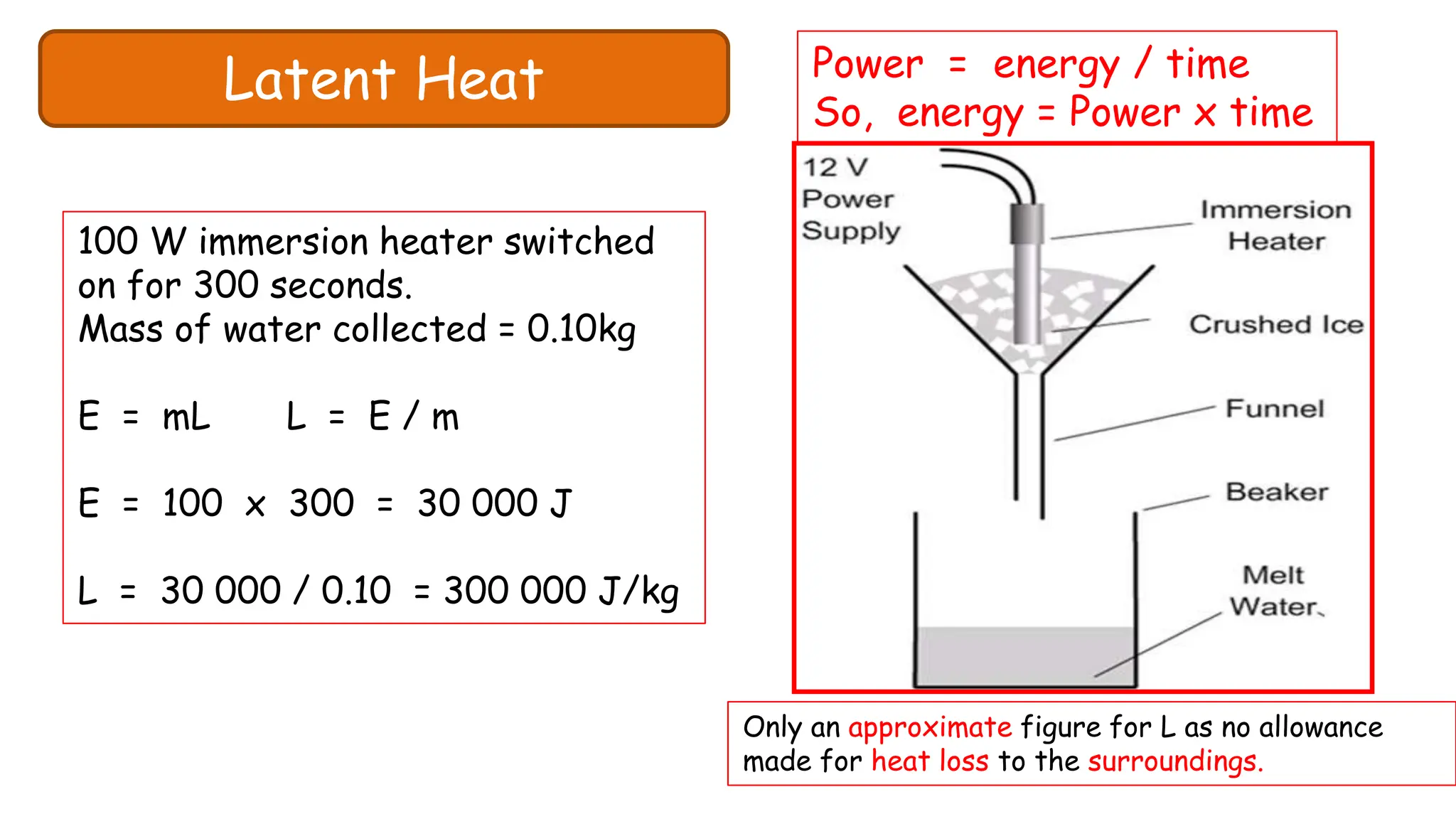
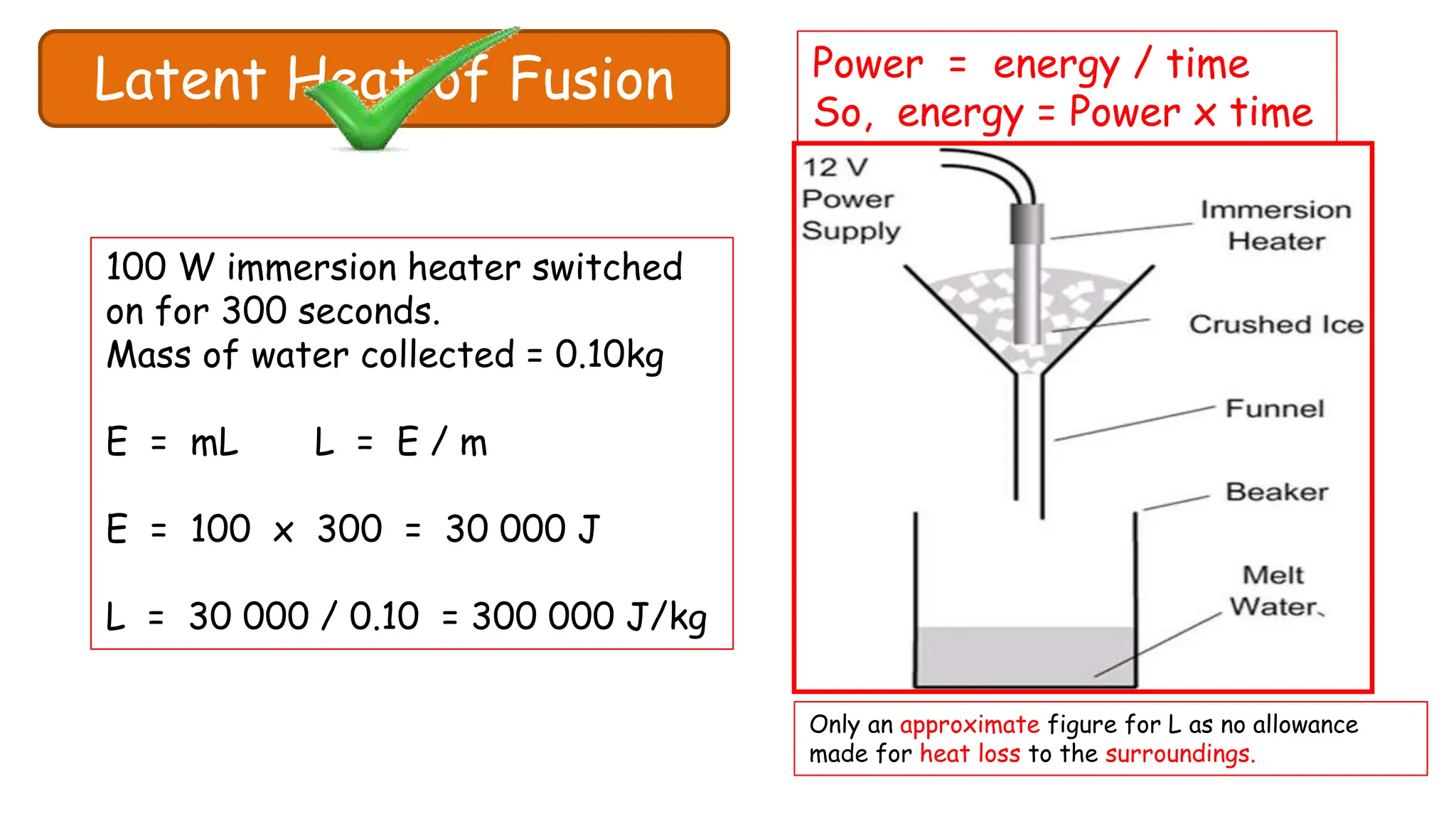
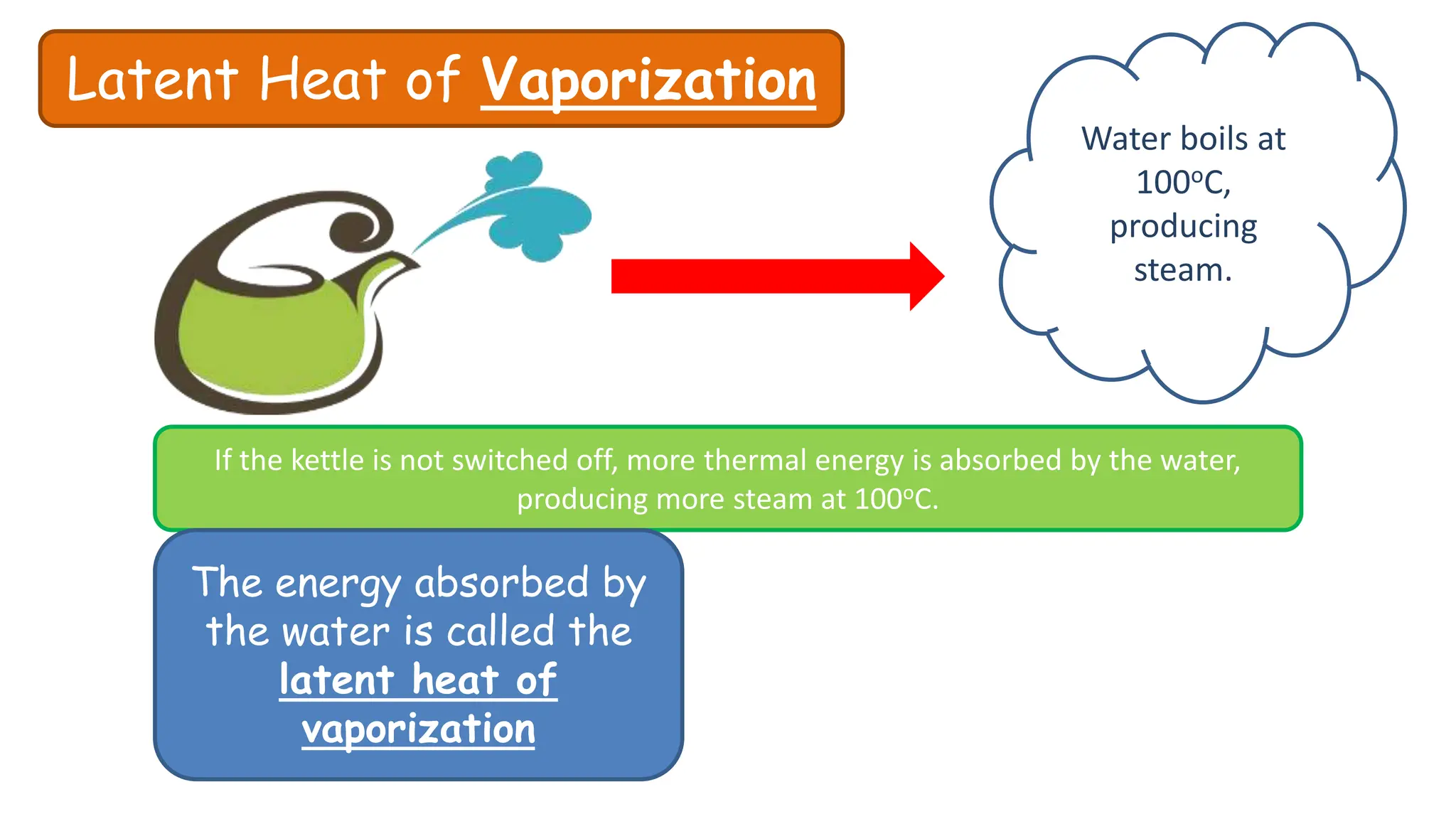
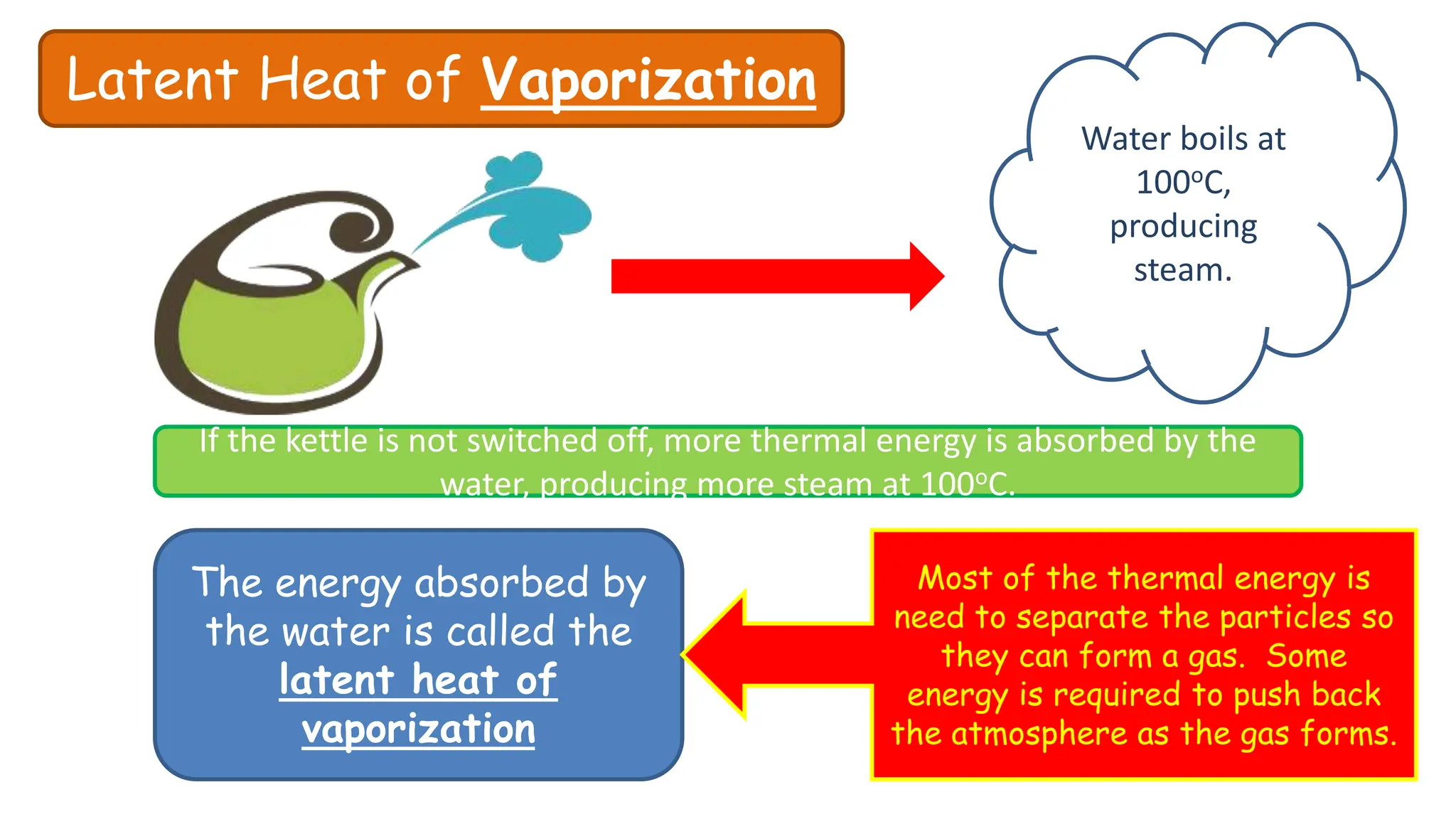
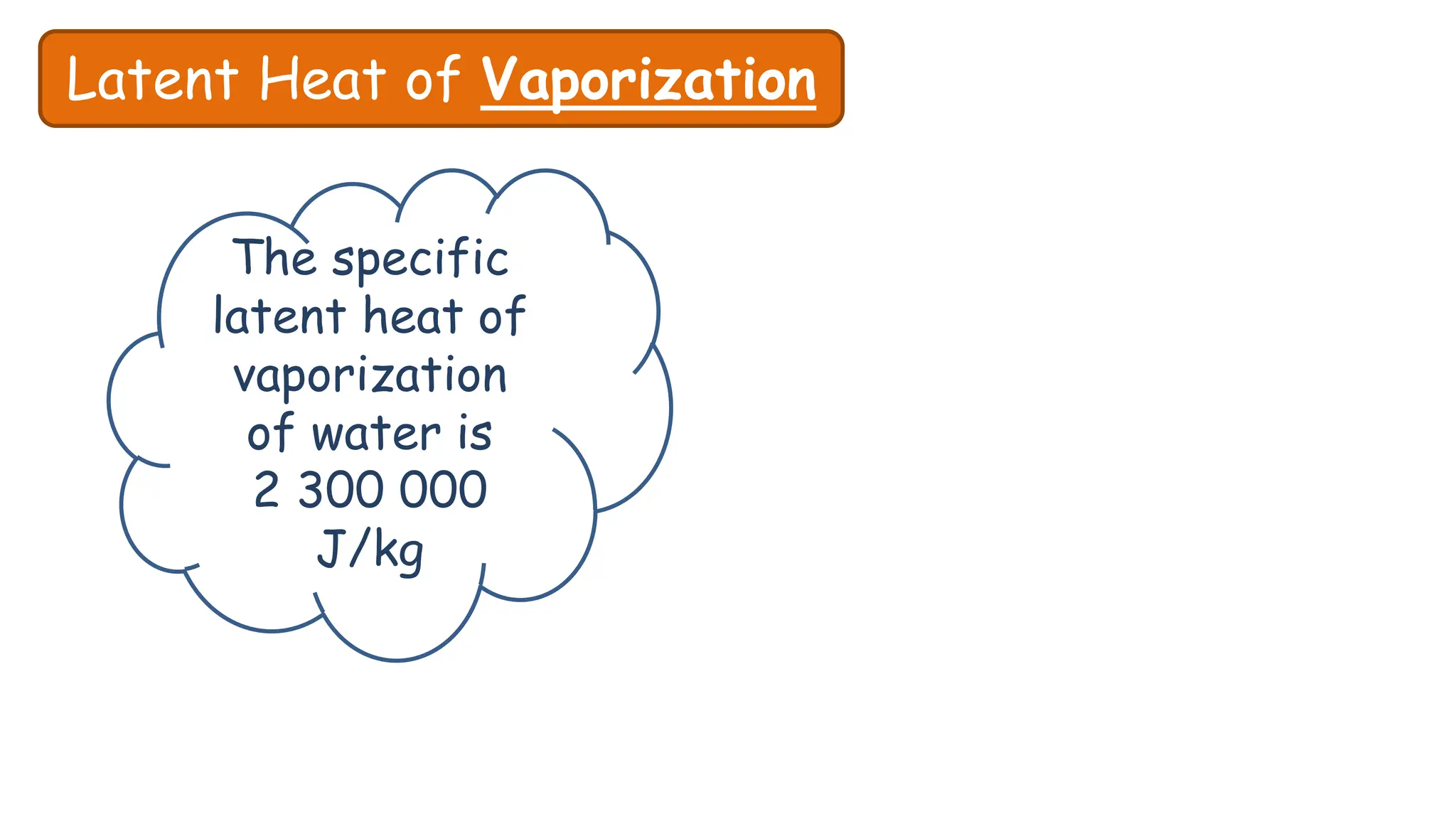
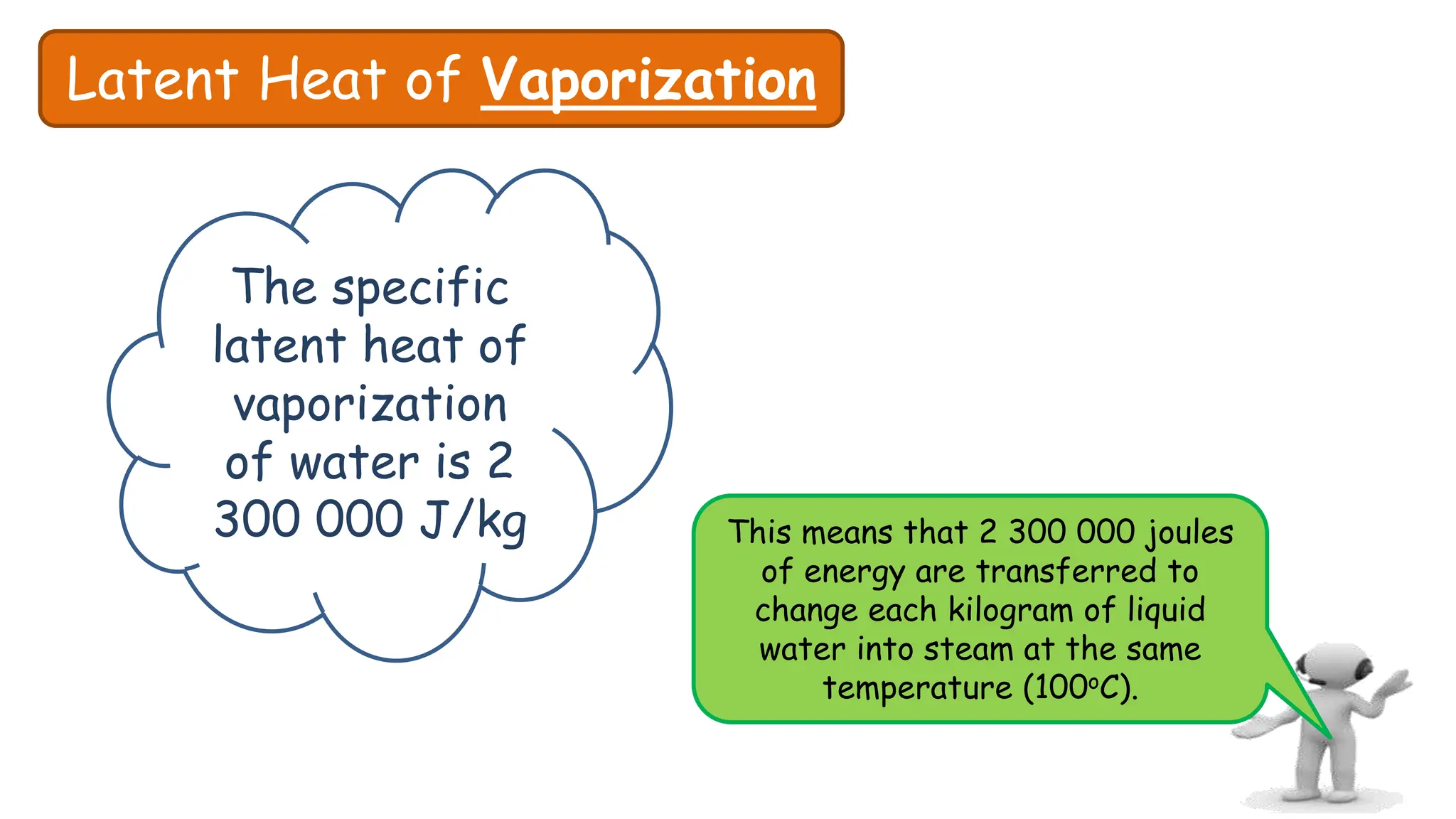
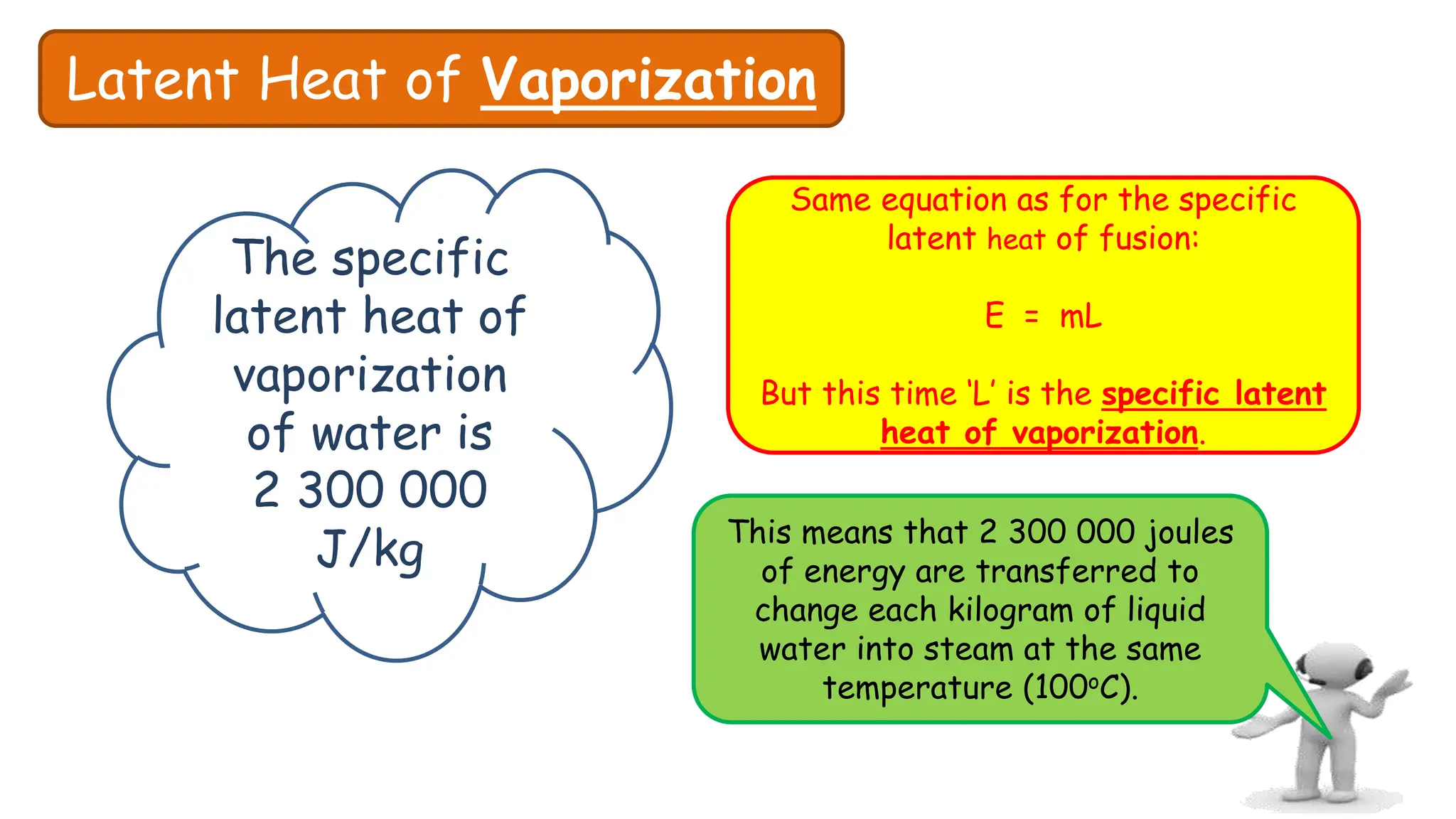
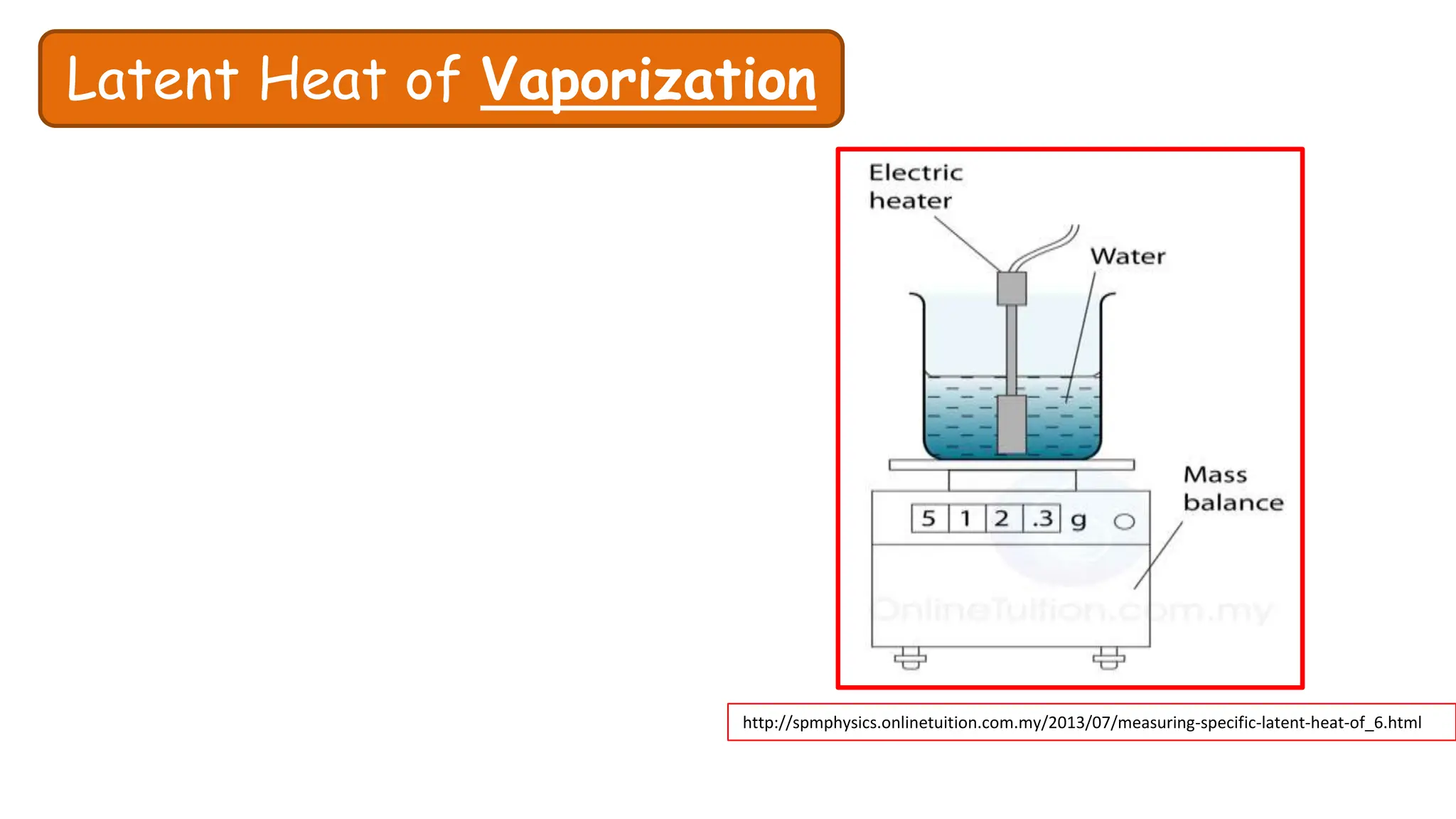
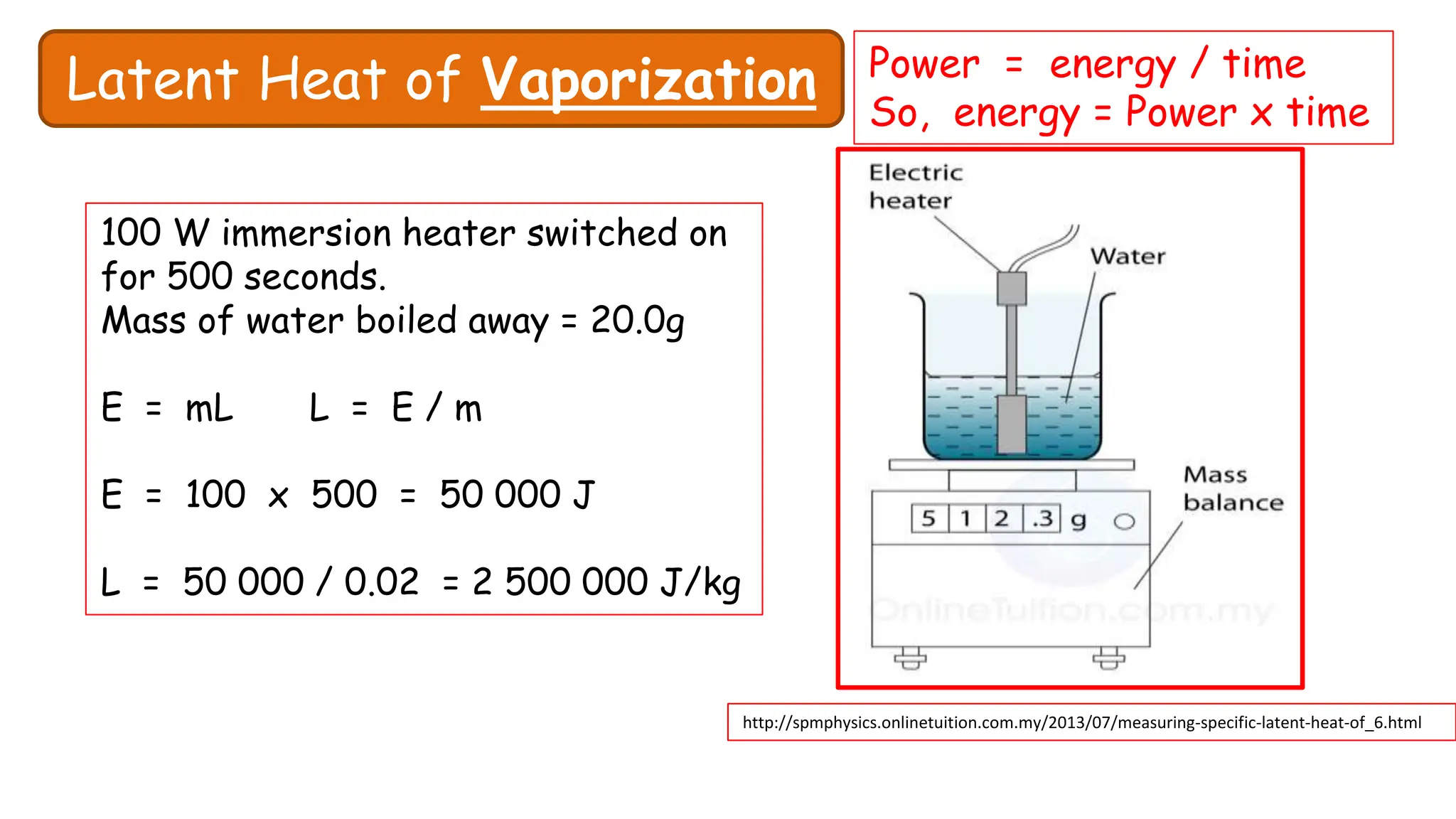
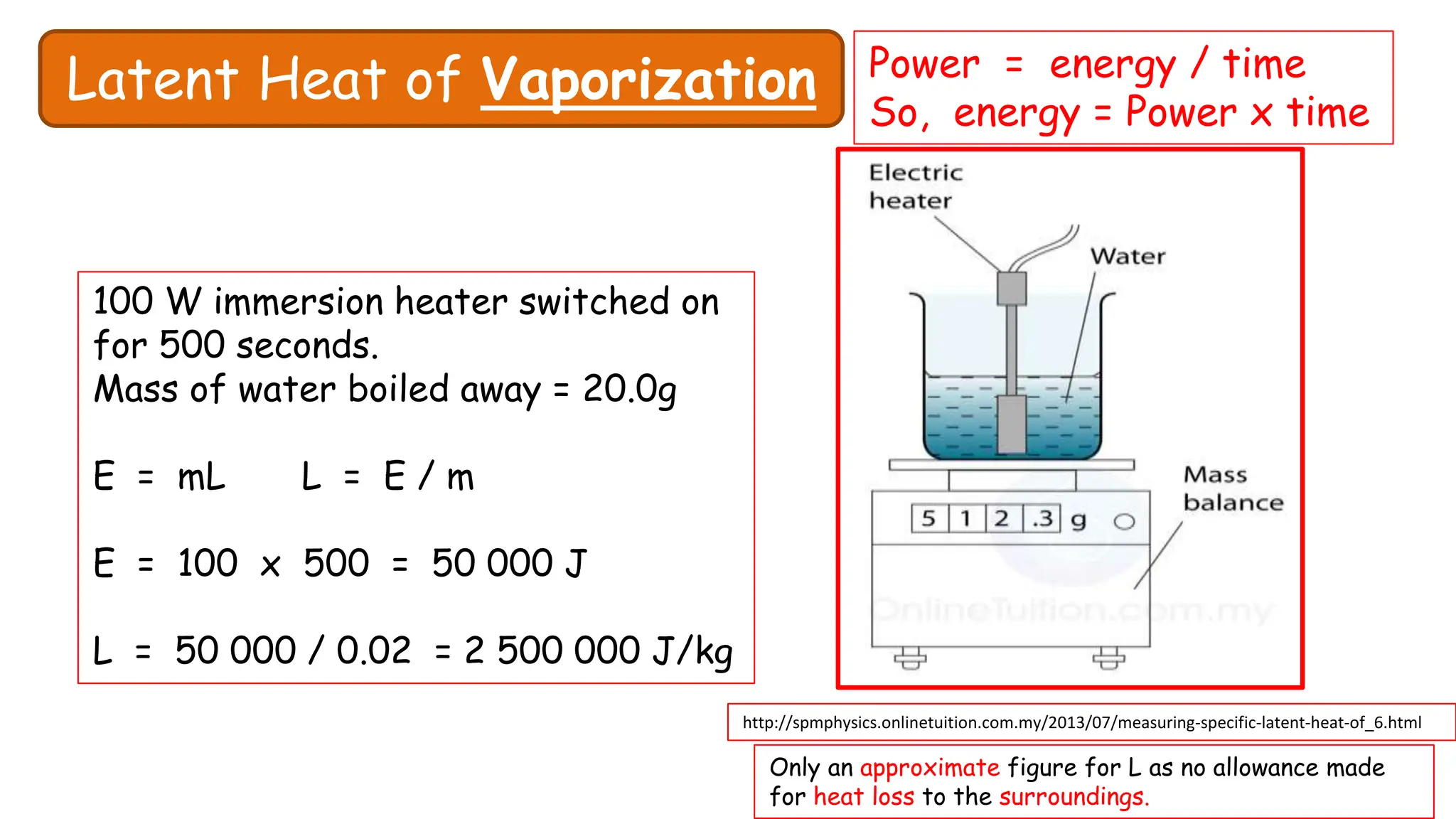
Chapter 6 discusses the concept of heat as energy transferred due to temperature differences, with units for measuring heat including erg, calorie, and joule. It explains the principles of thermal equilibrium, specific heat capacity, and the effects of heat on matter, alongside practical applications and comparisons between heat and temperature. Additionally, the chapter covers latent heat related to phase changes, defining the specific latent heat of fusion and vaporization of water.