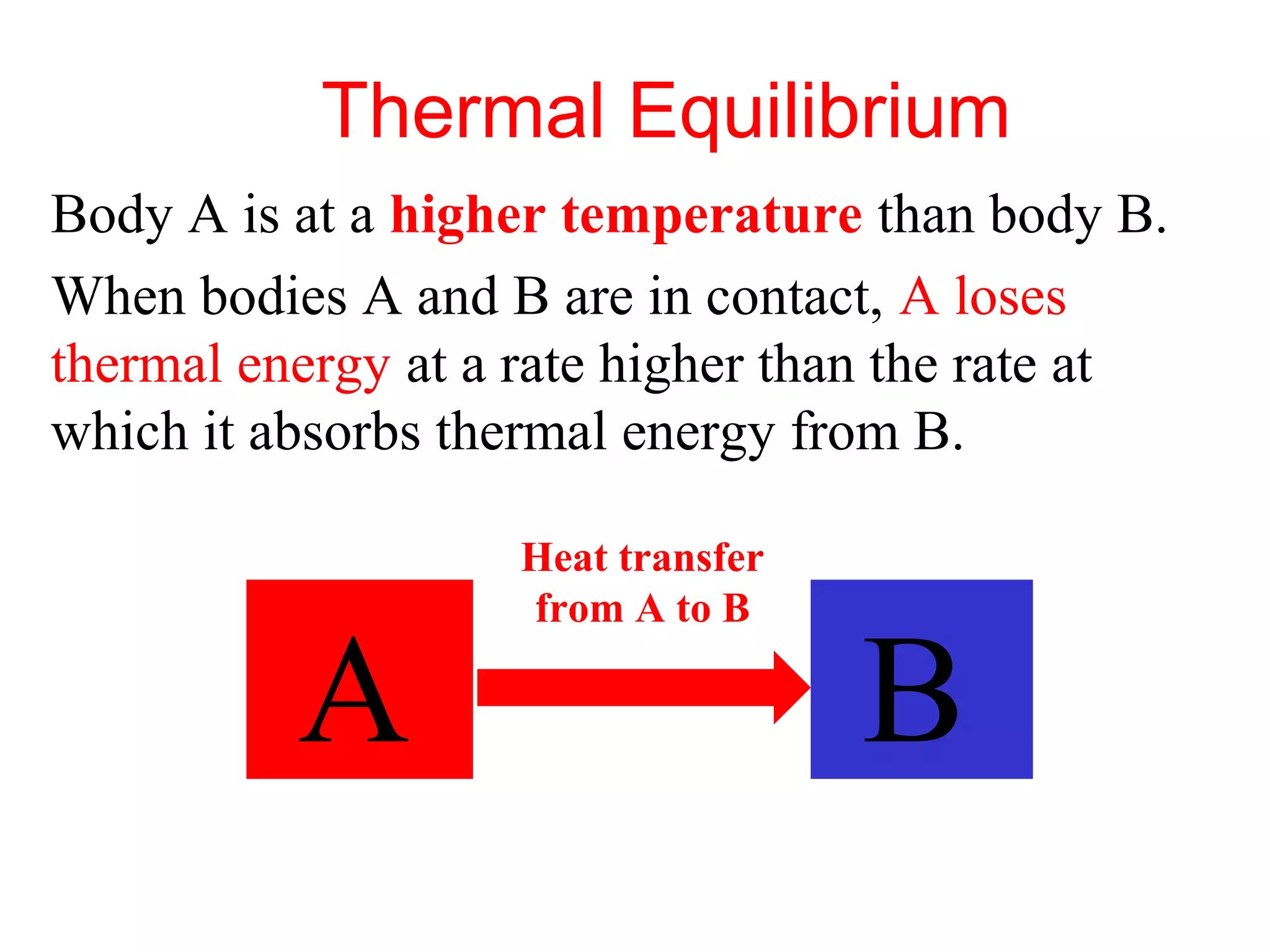
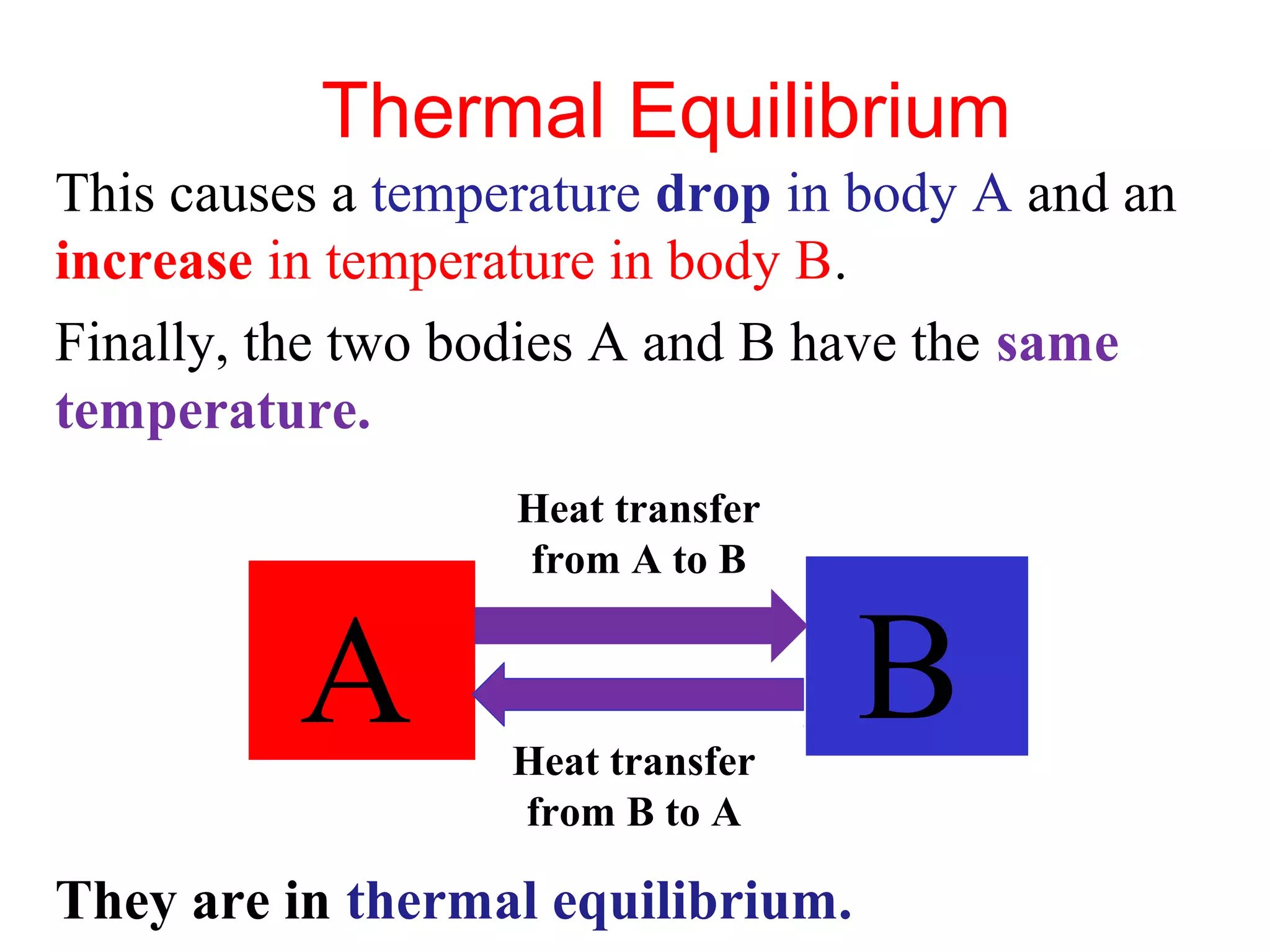
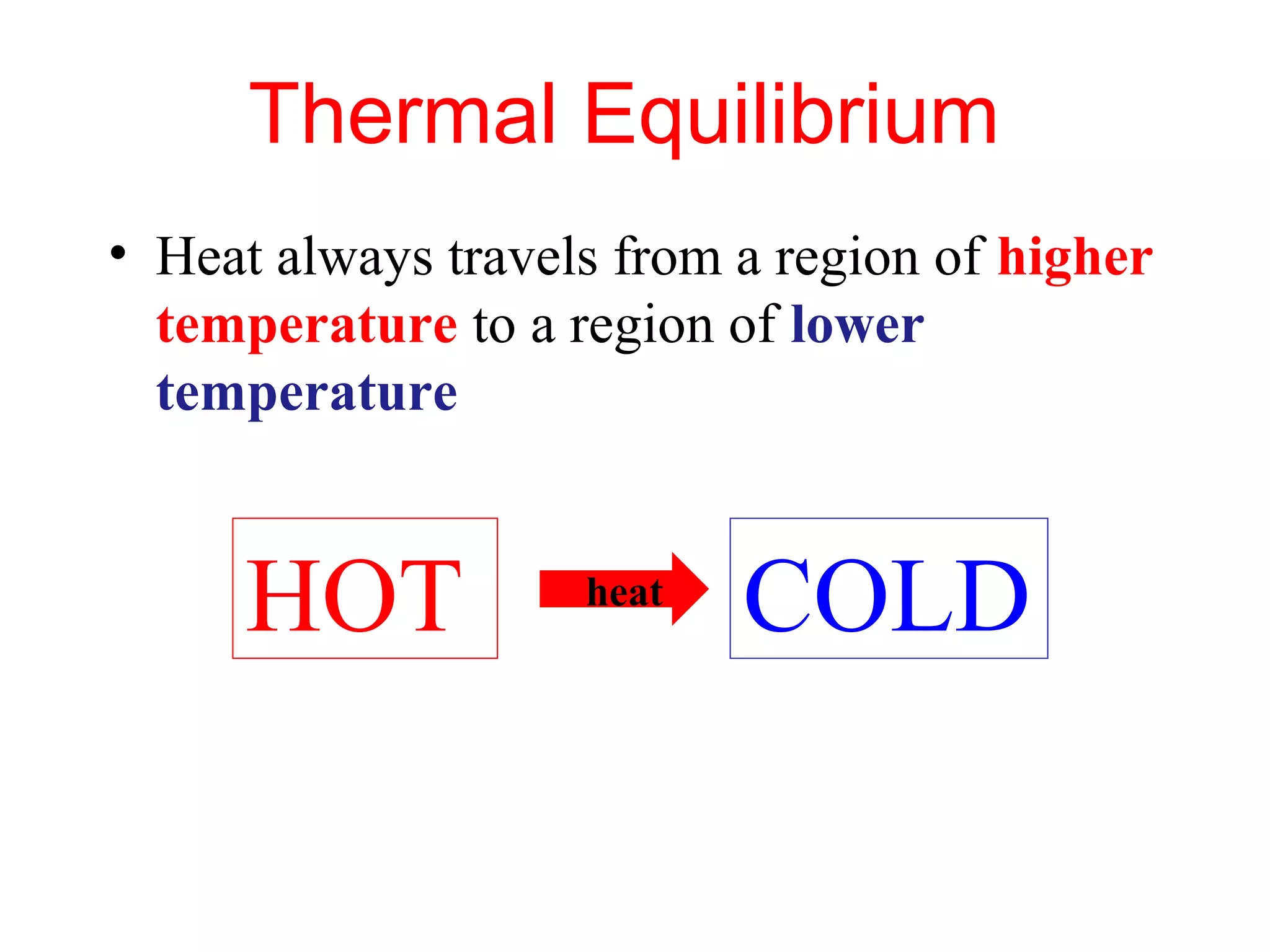
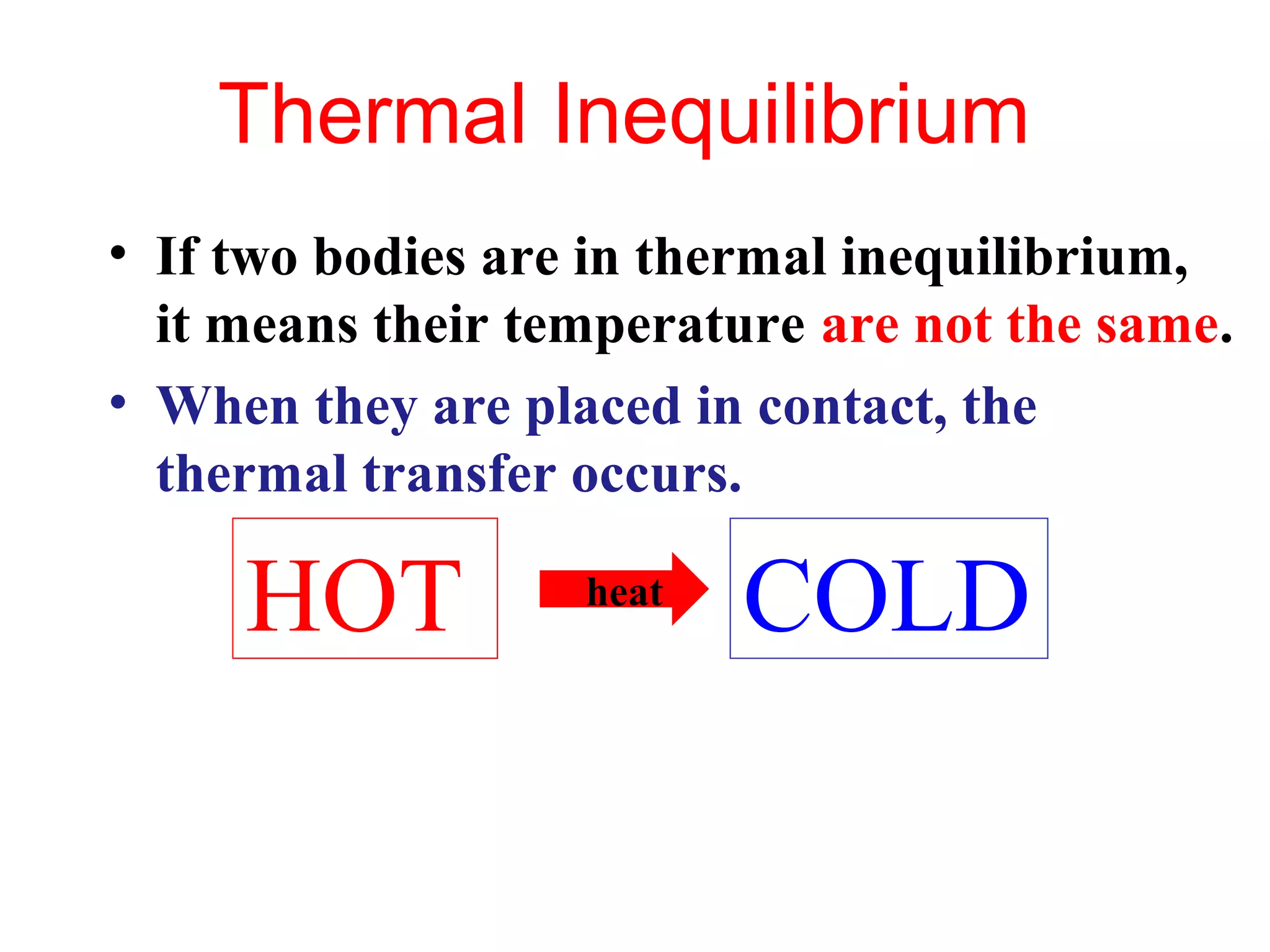
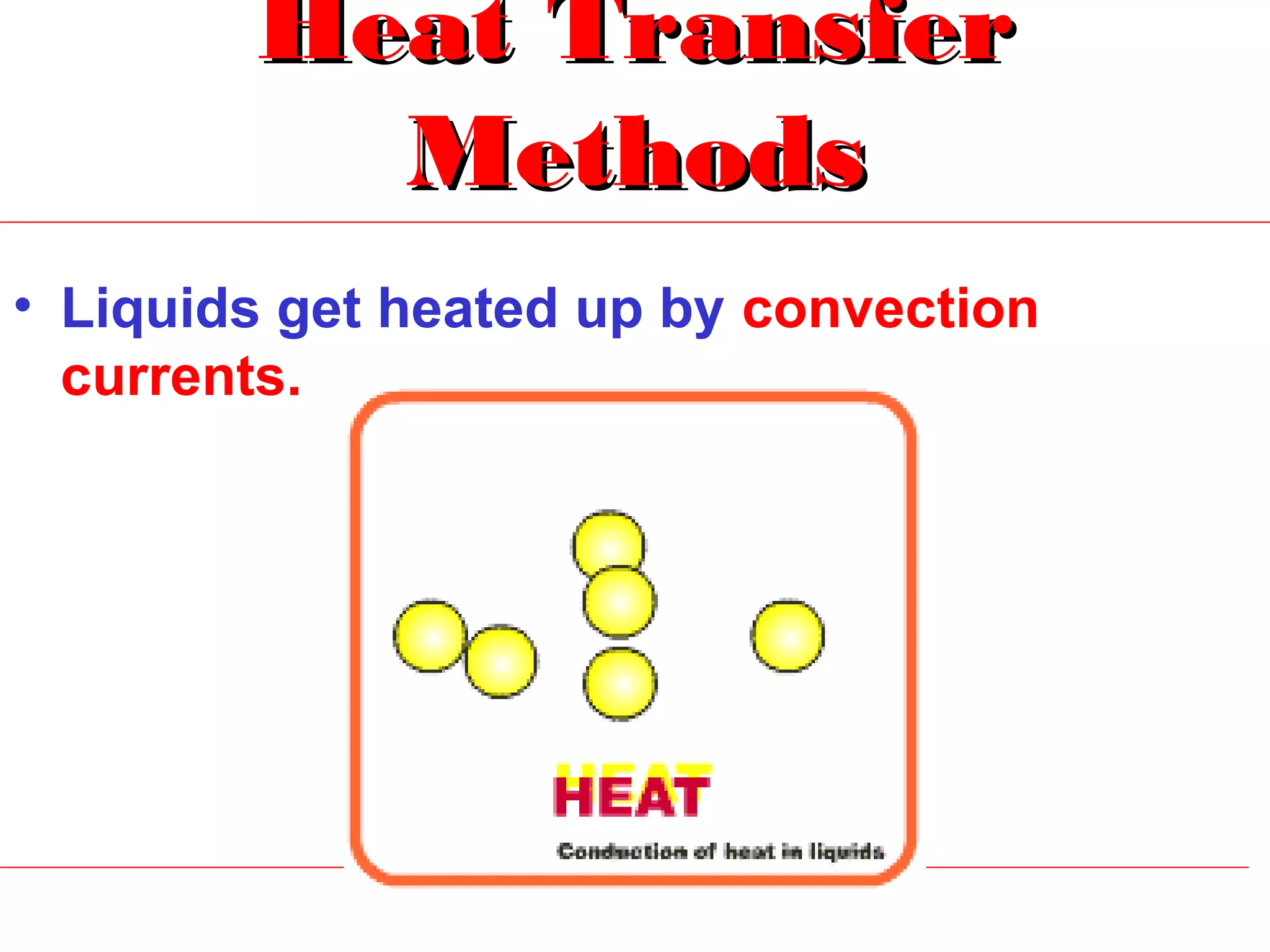
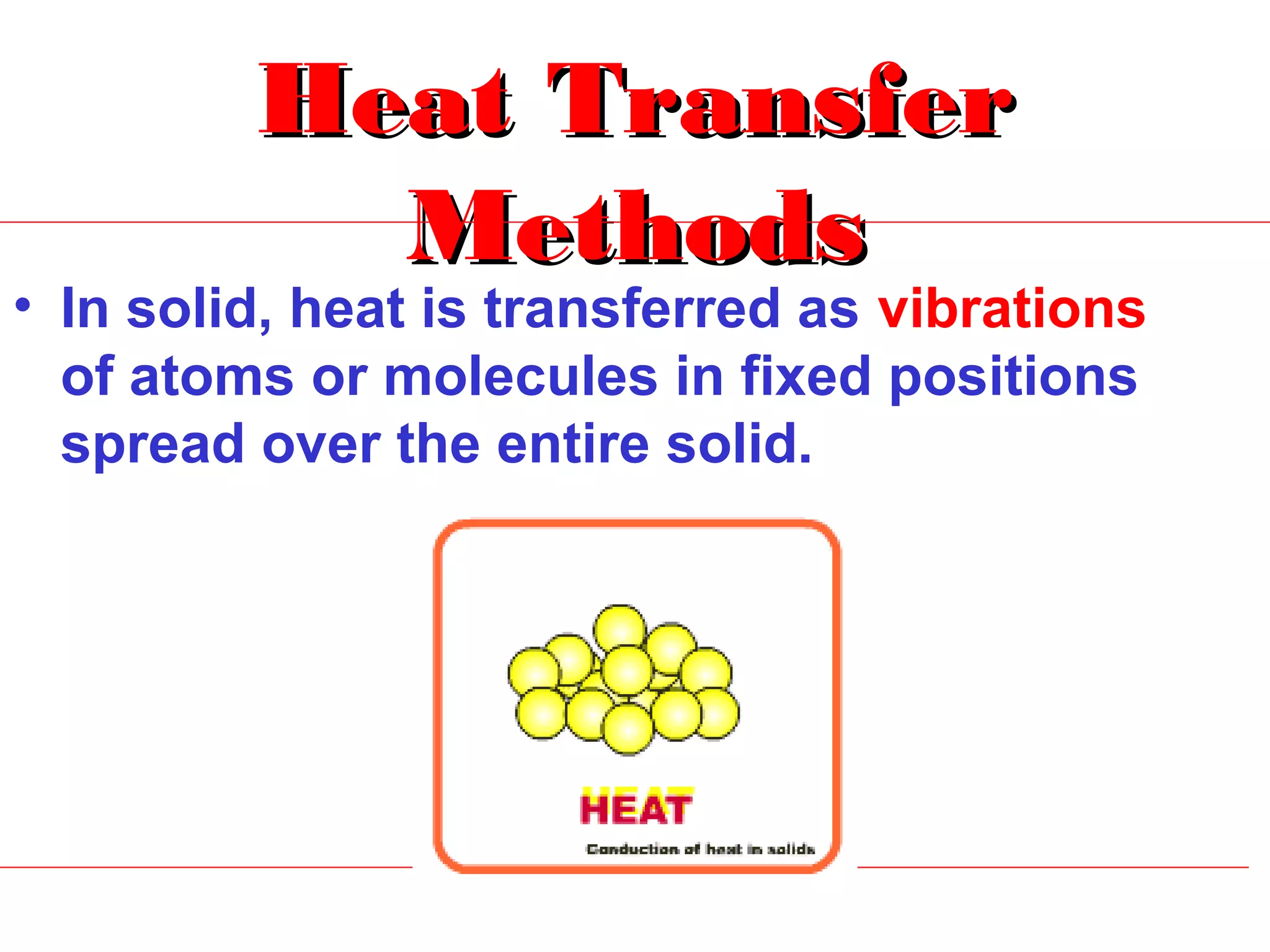
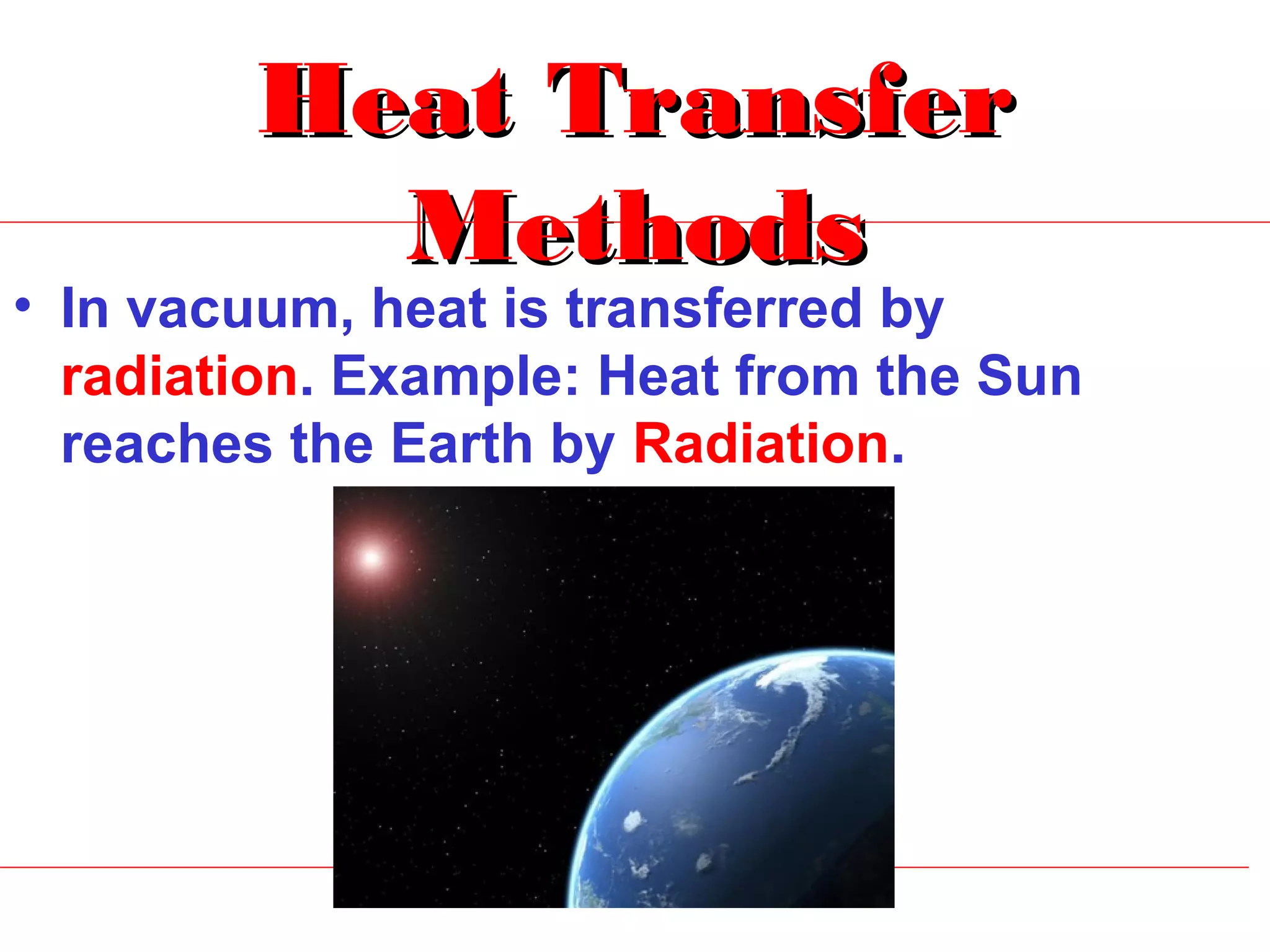
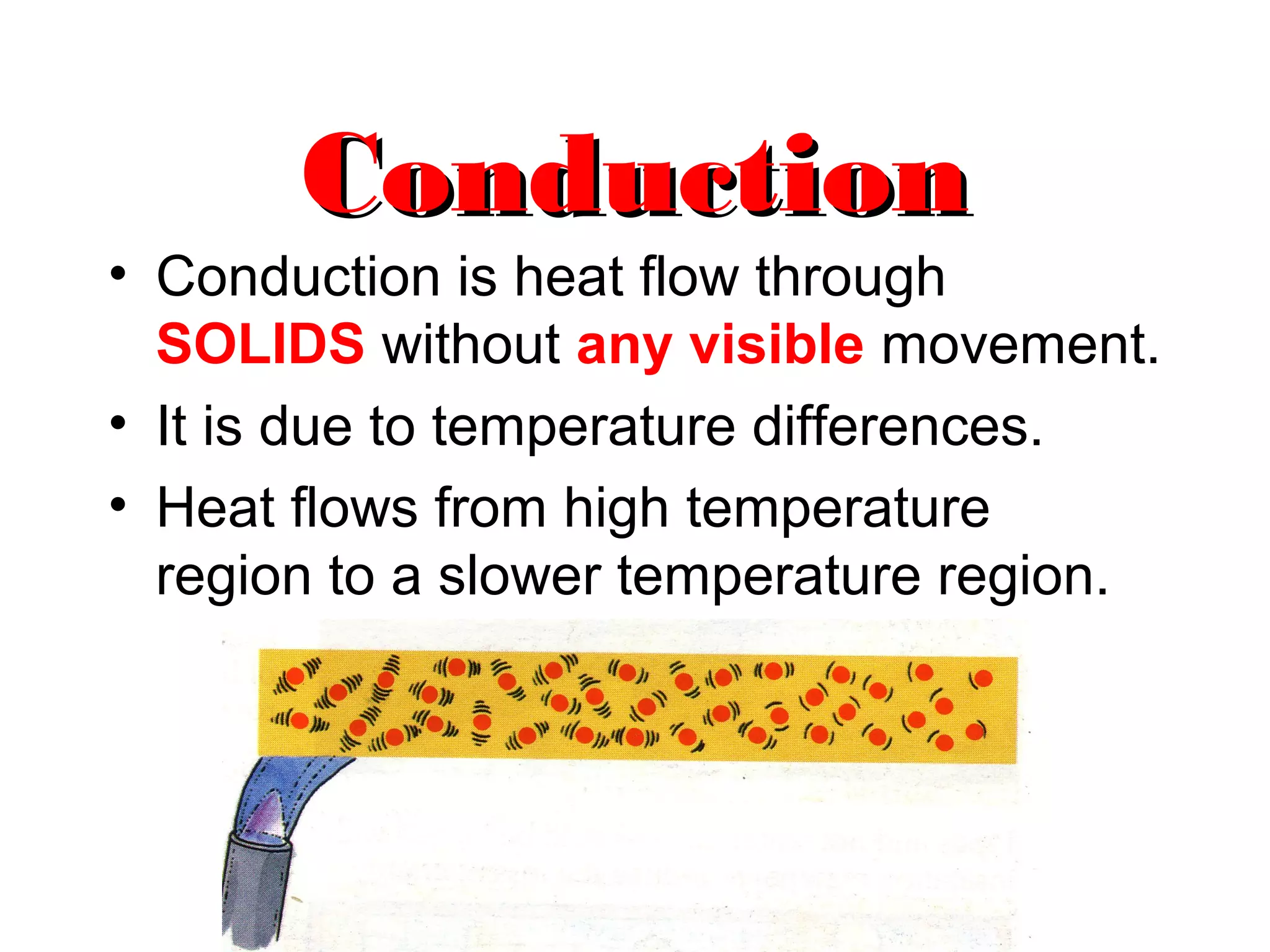
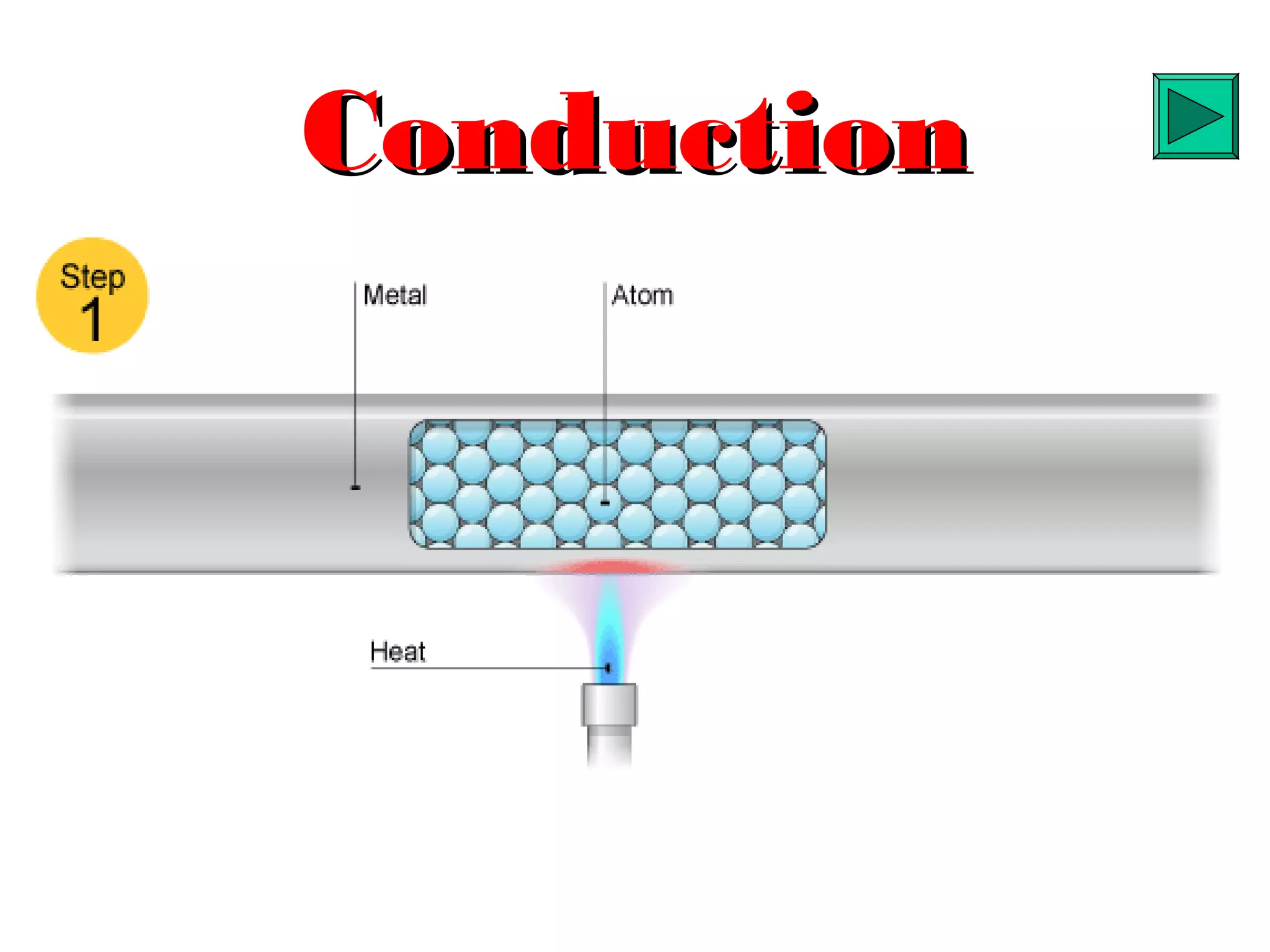
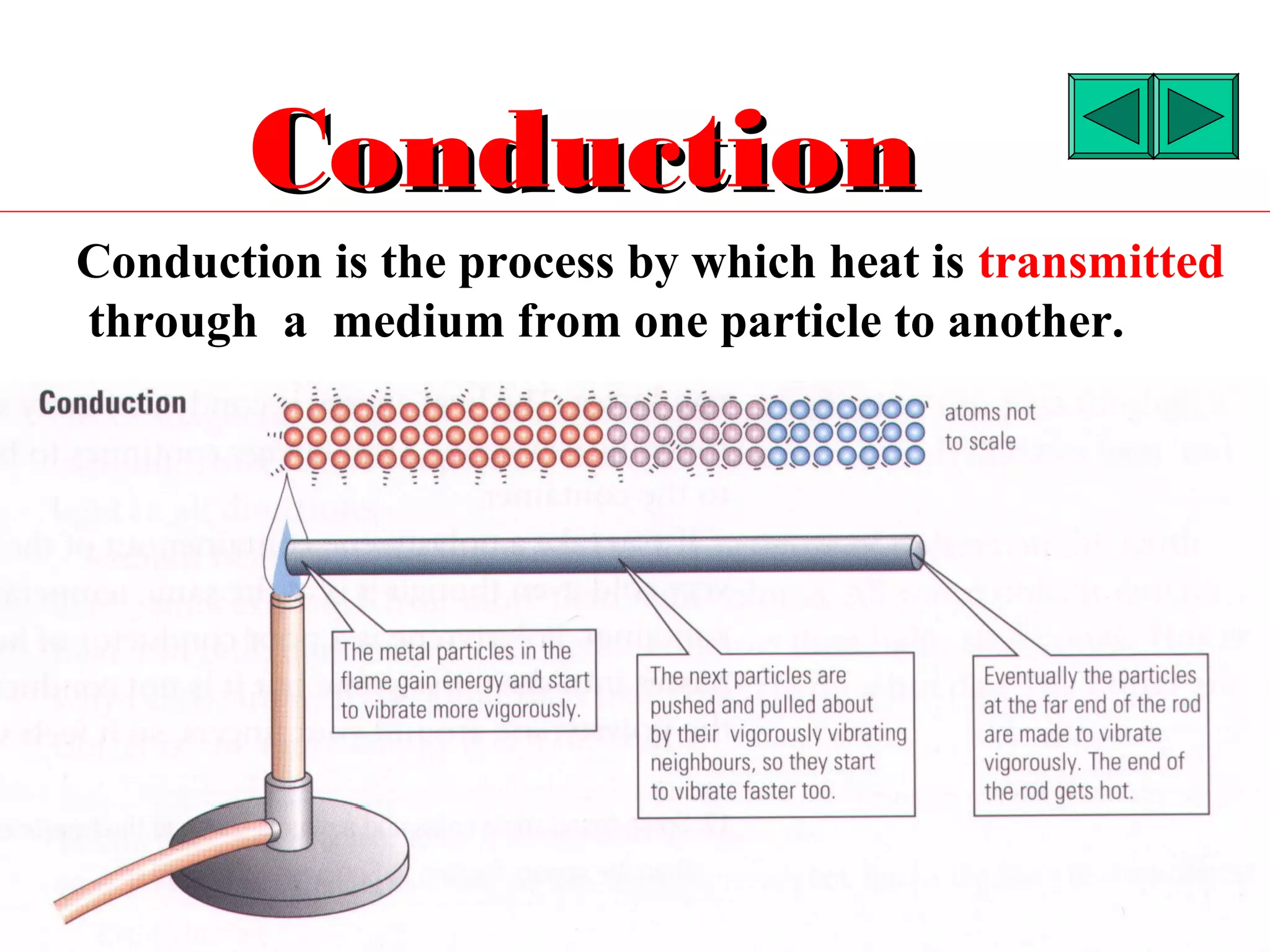
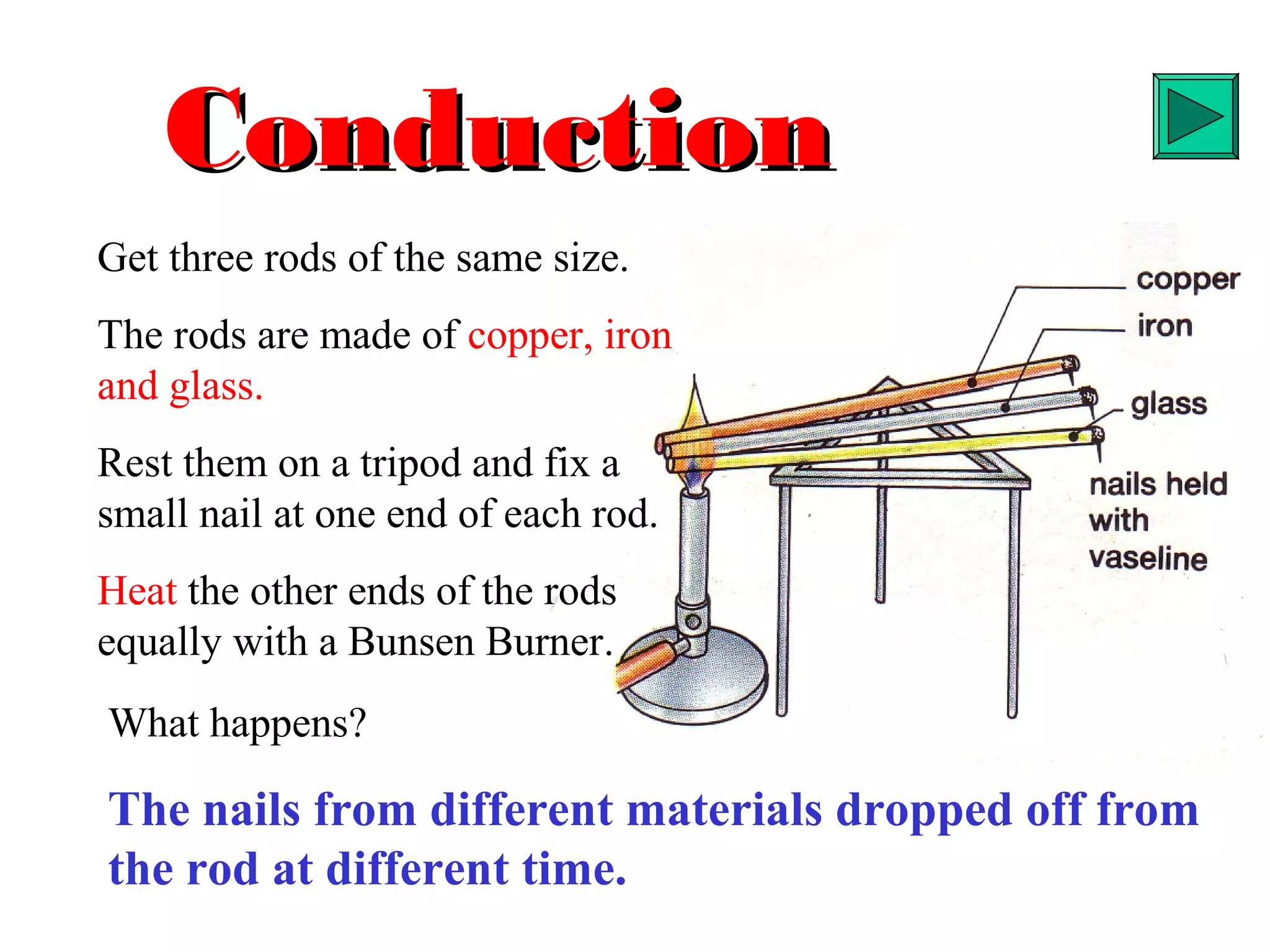
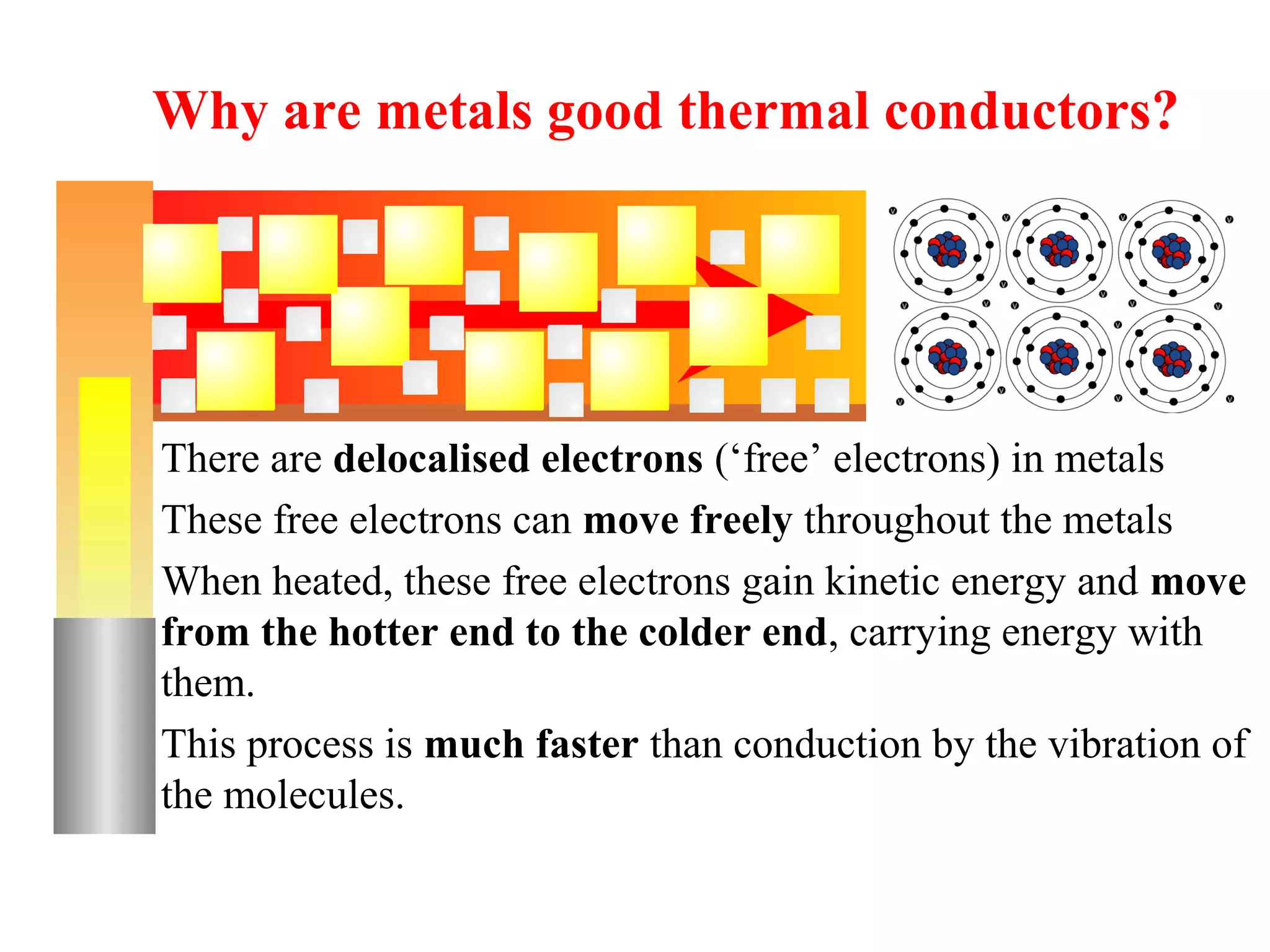
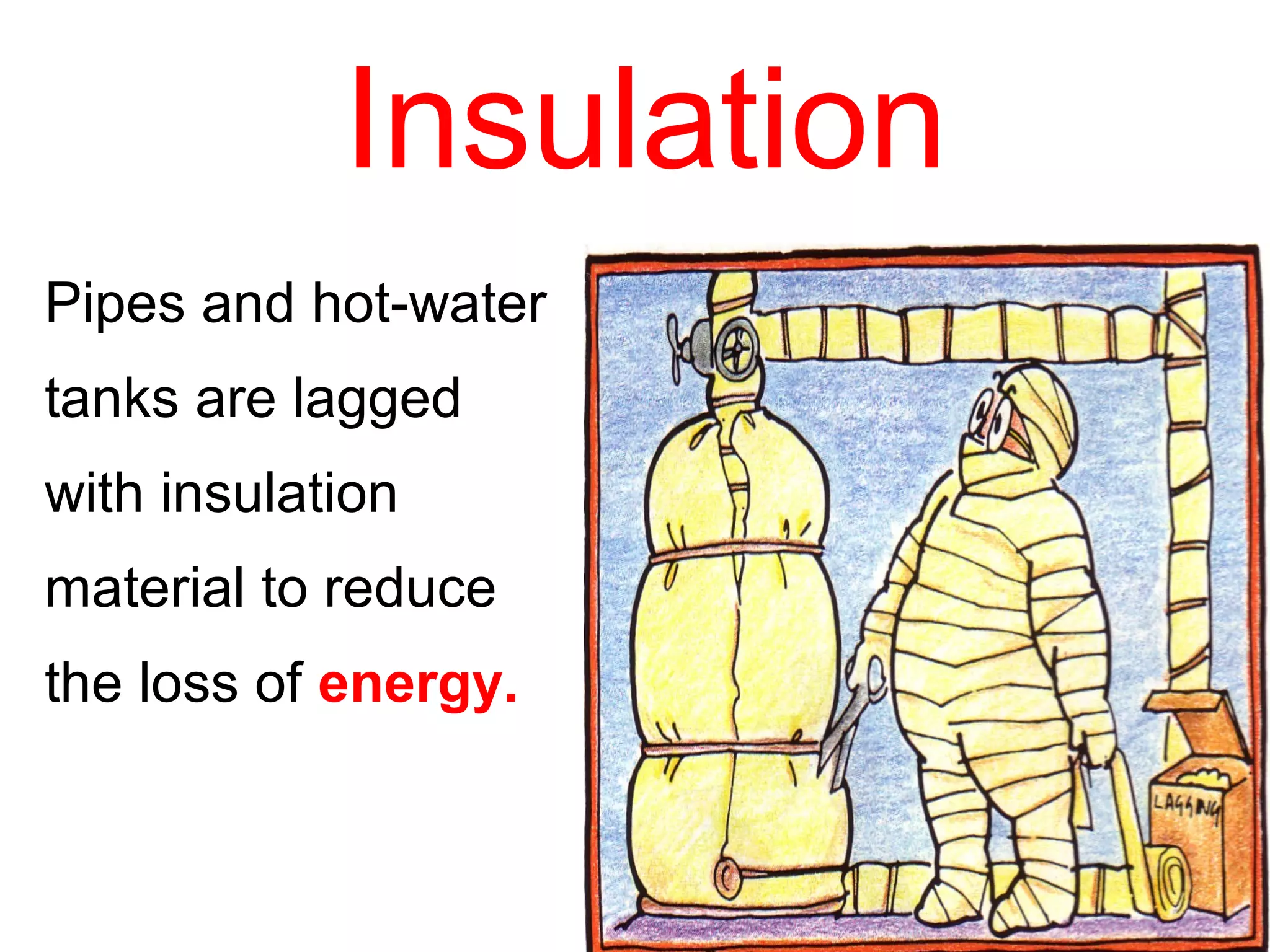
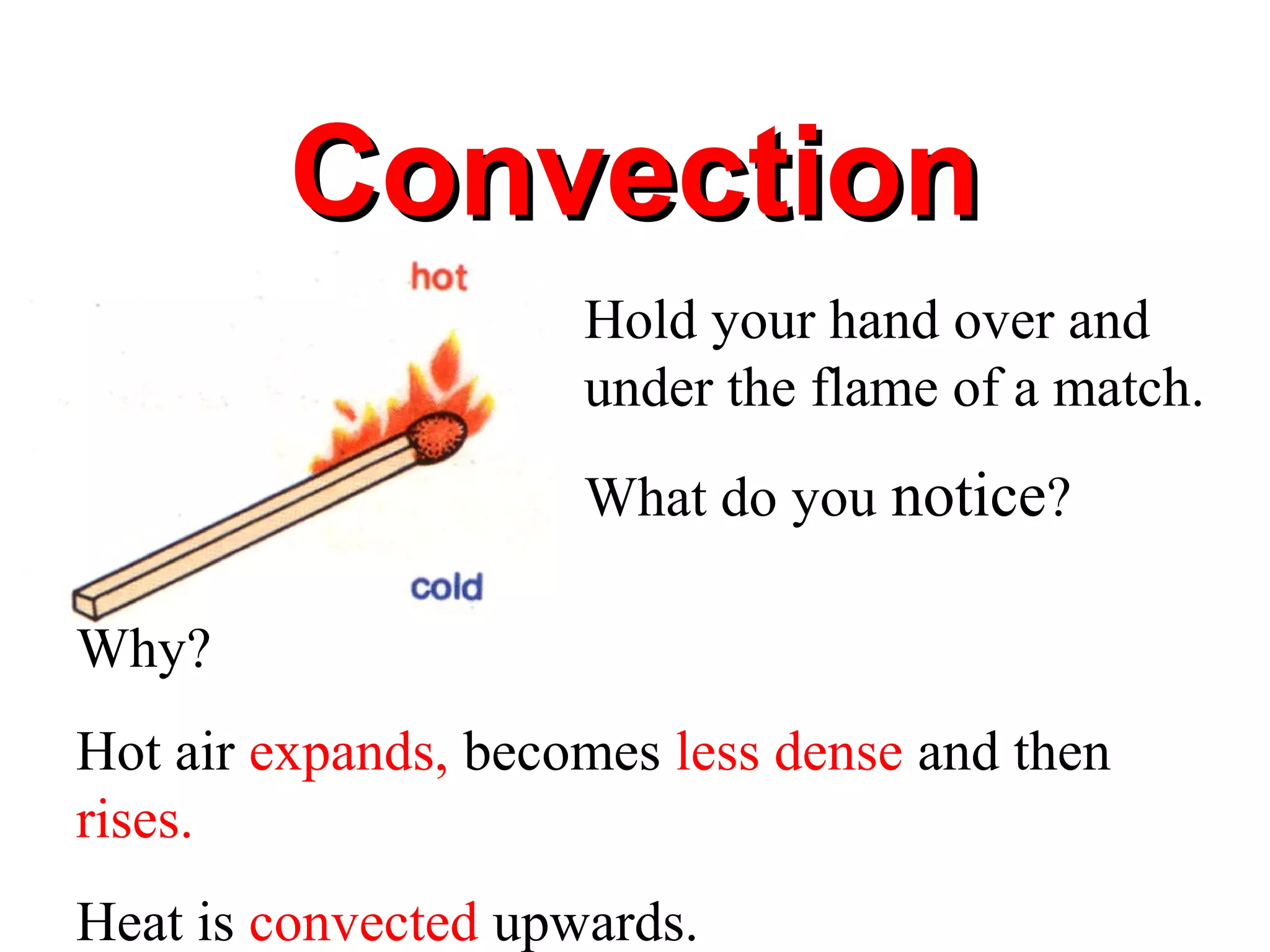
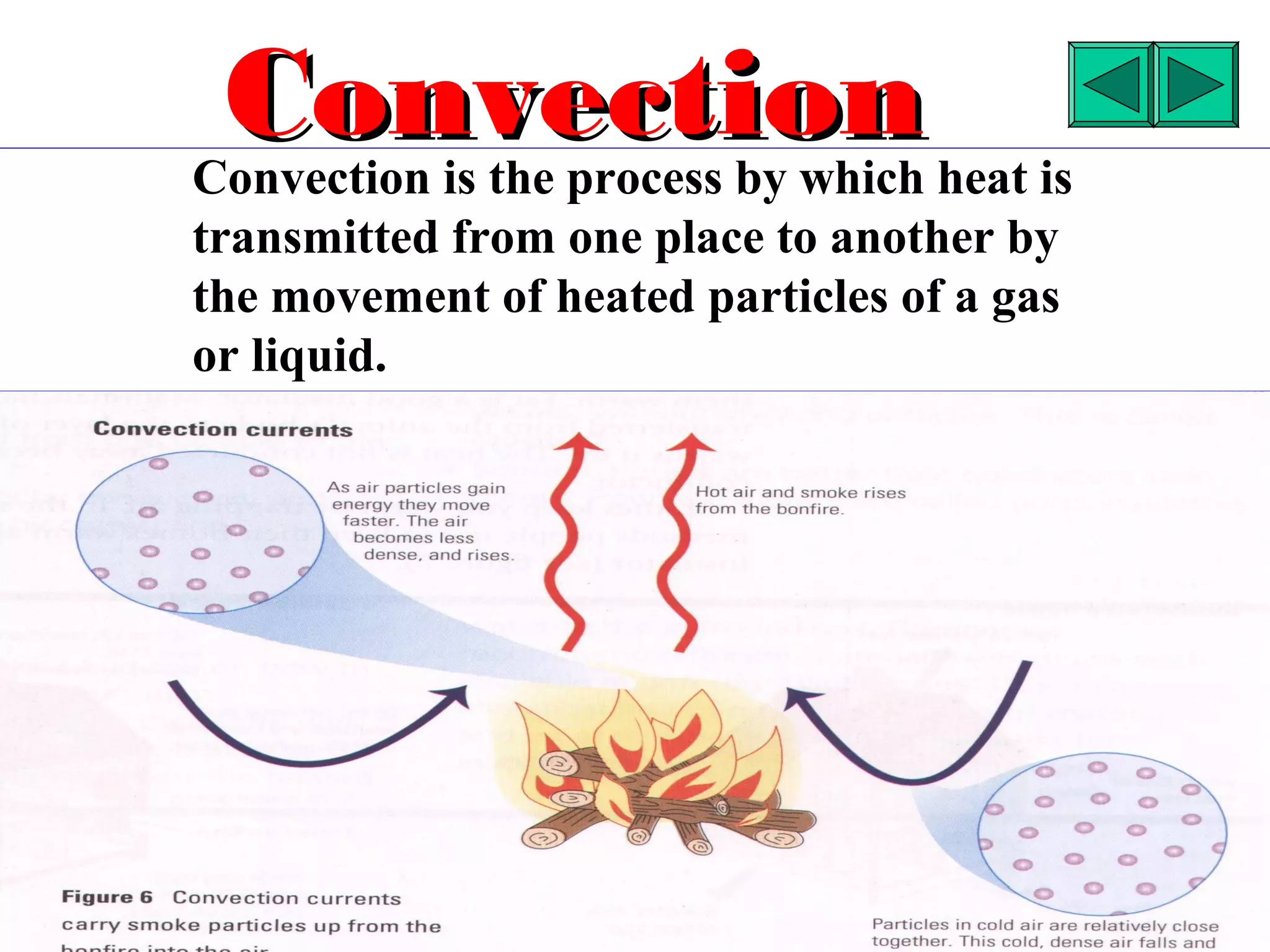
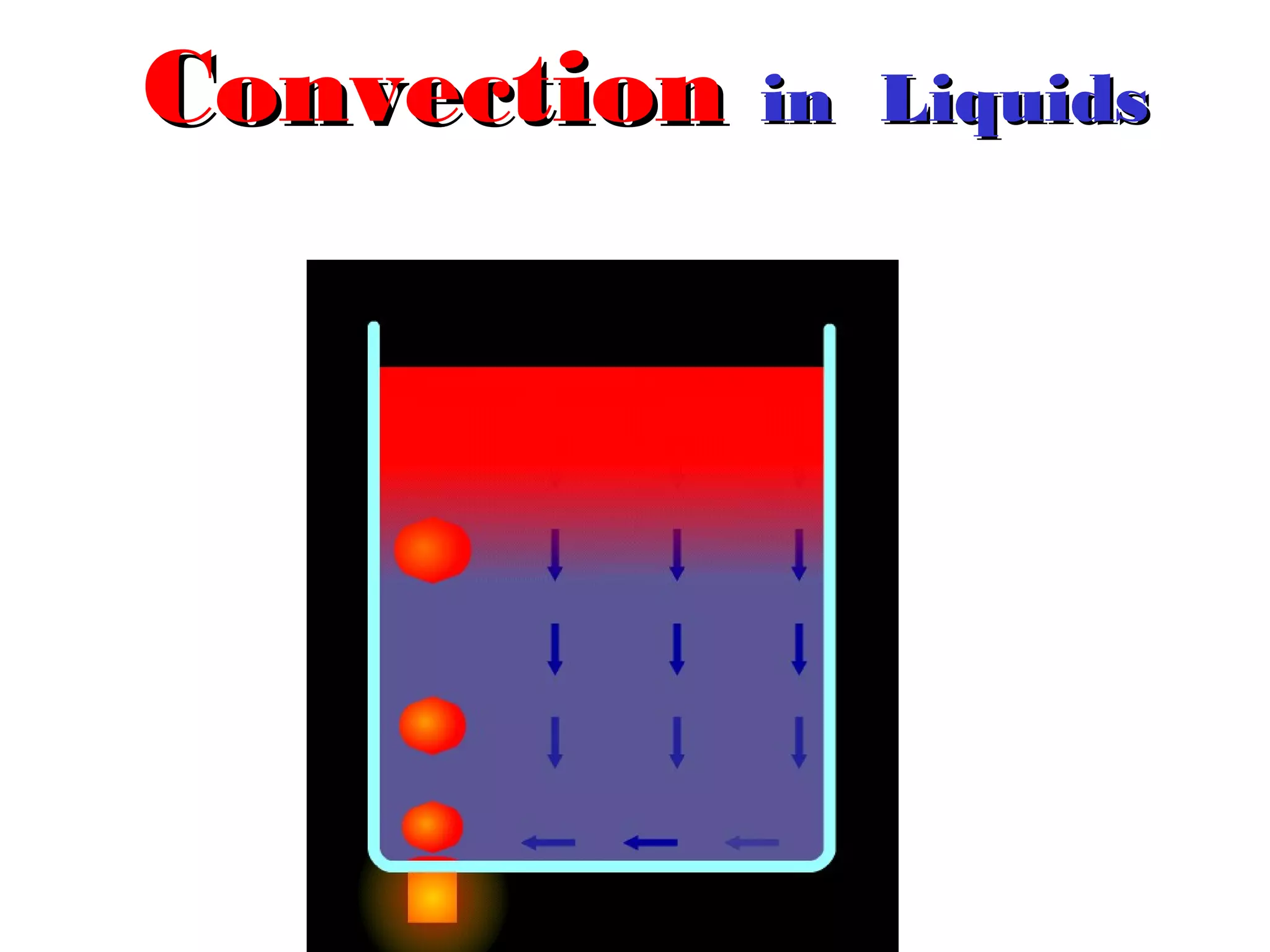
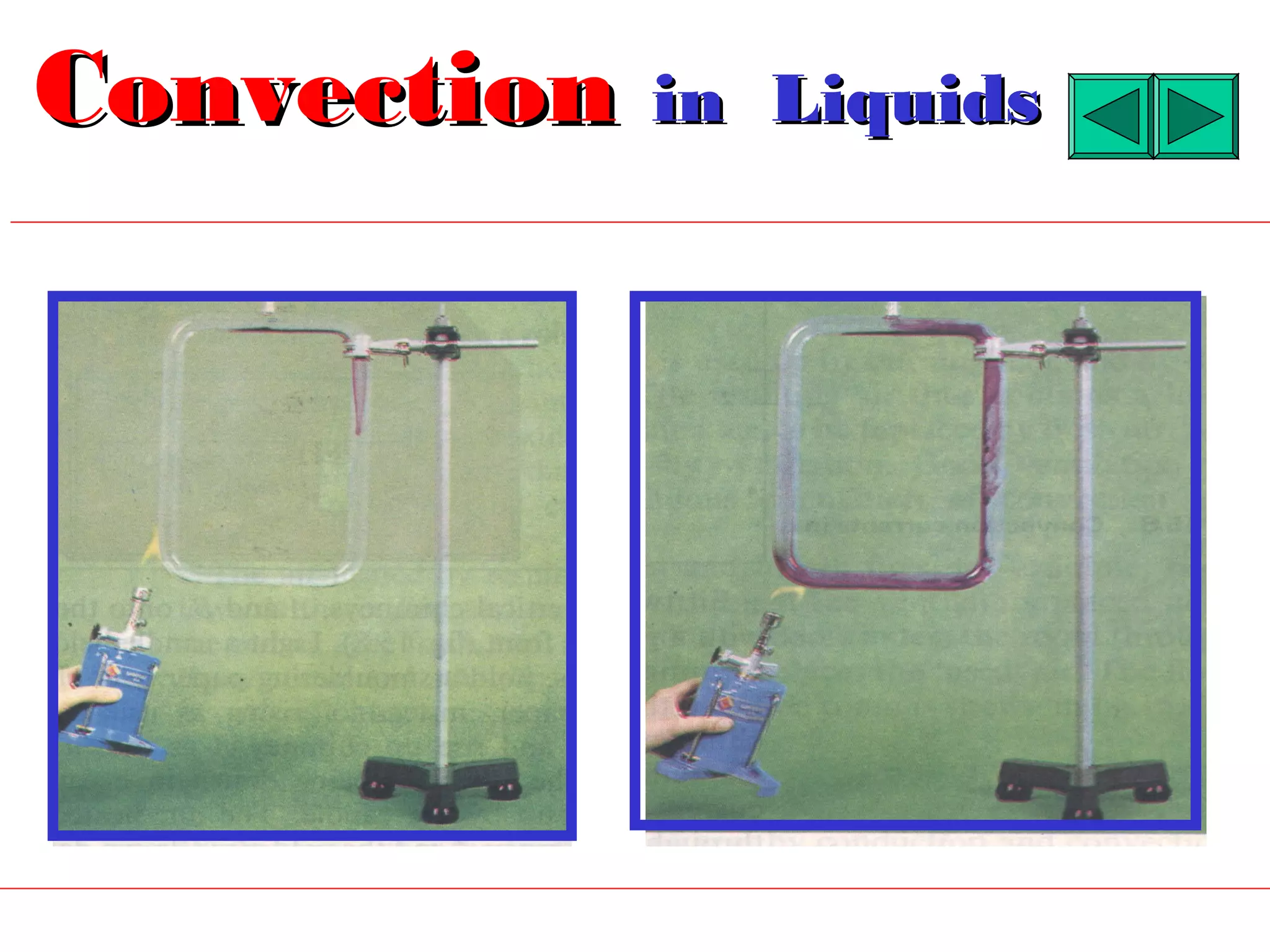
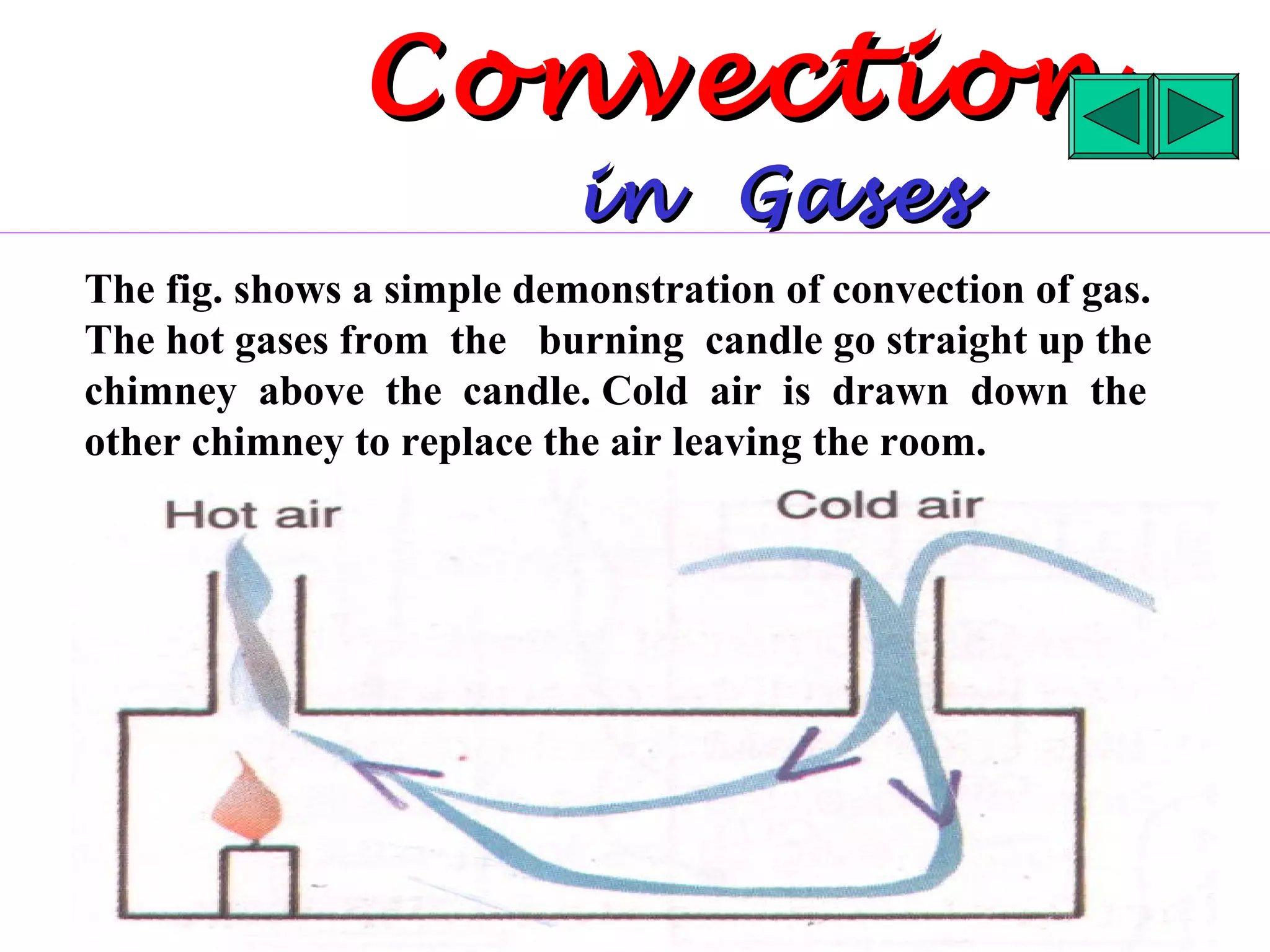
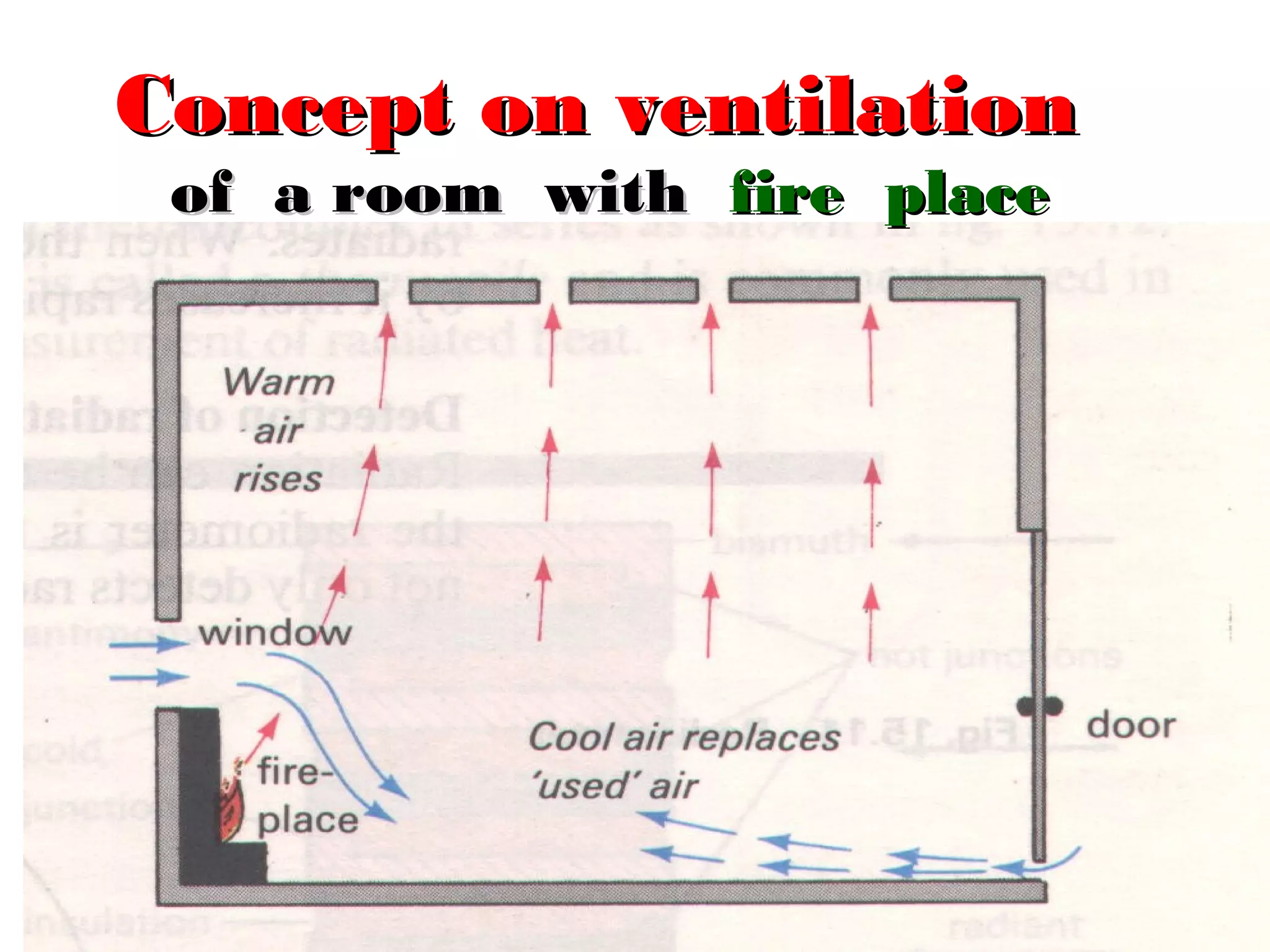
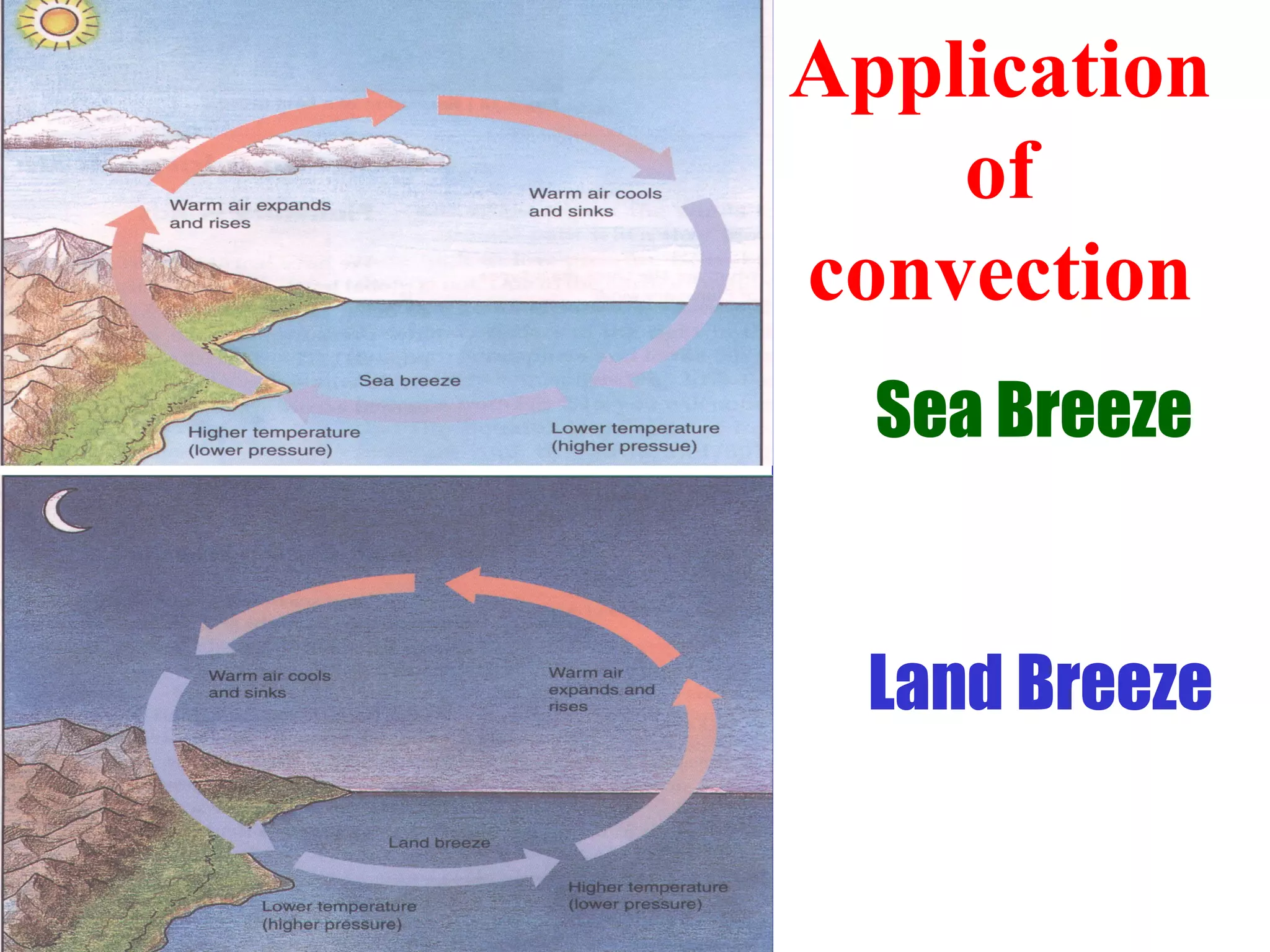
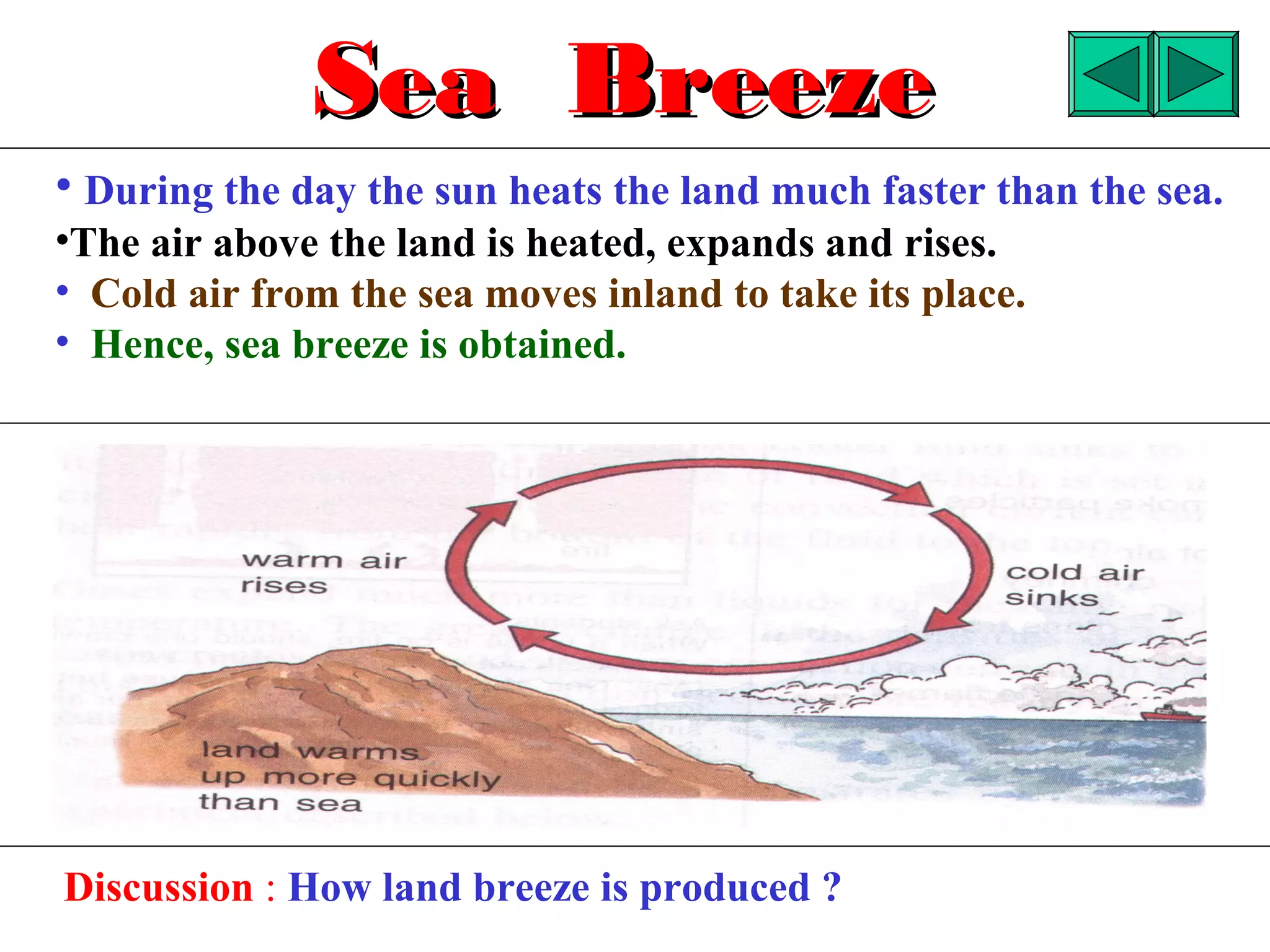
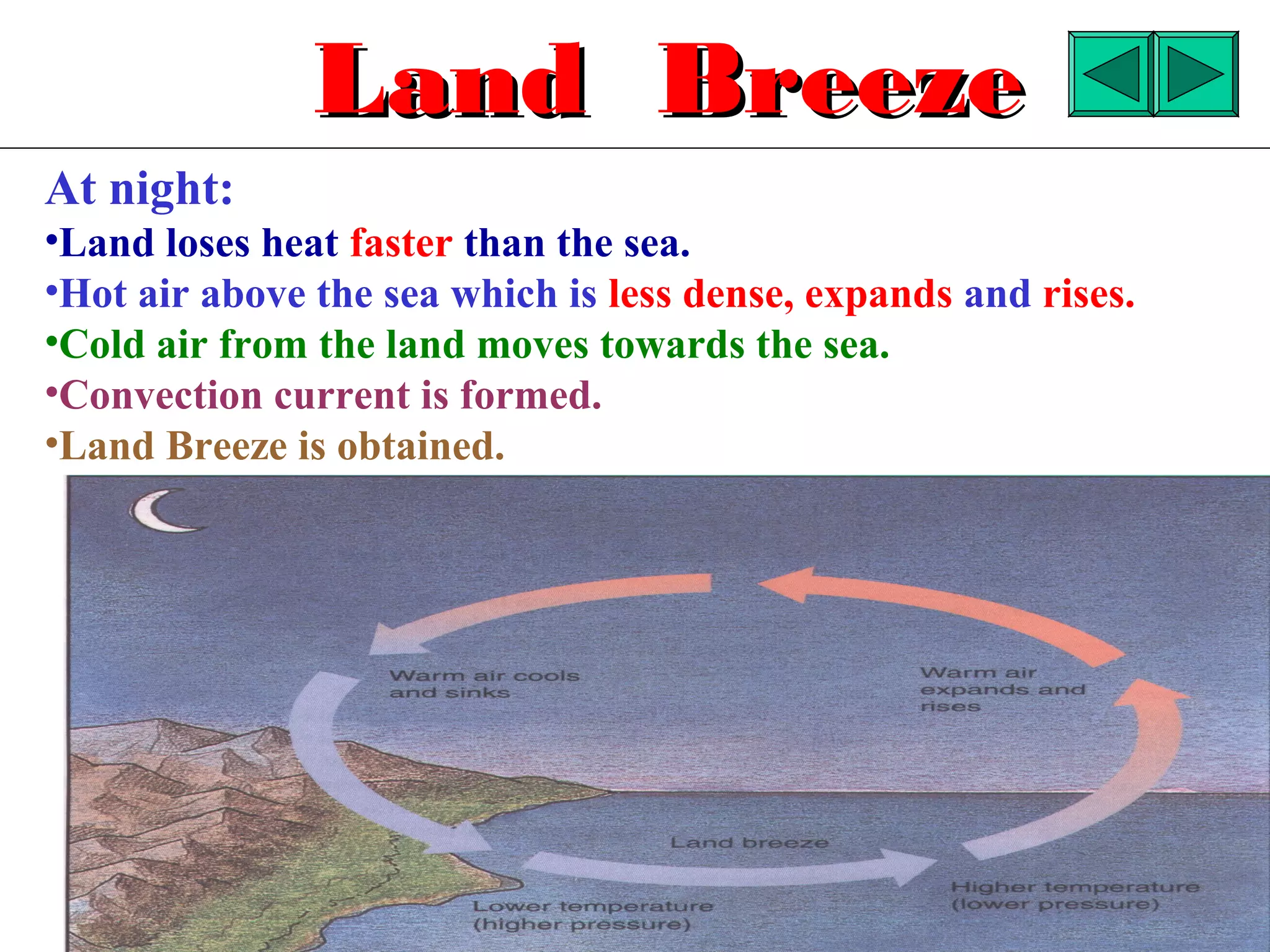
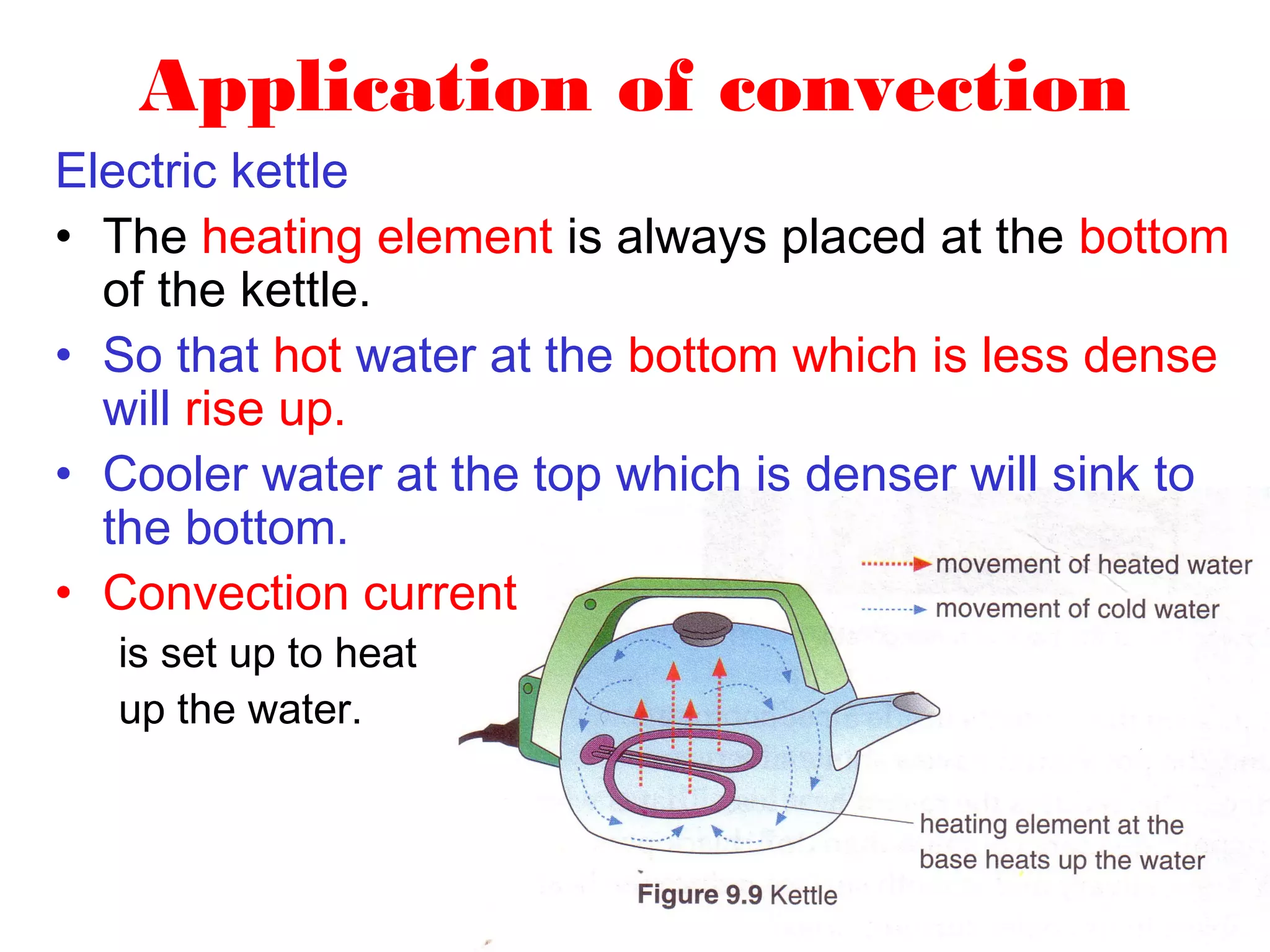
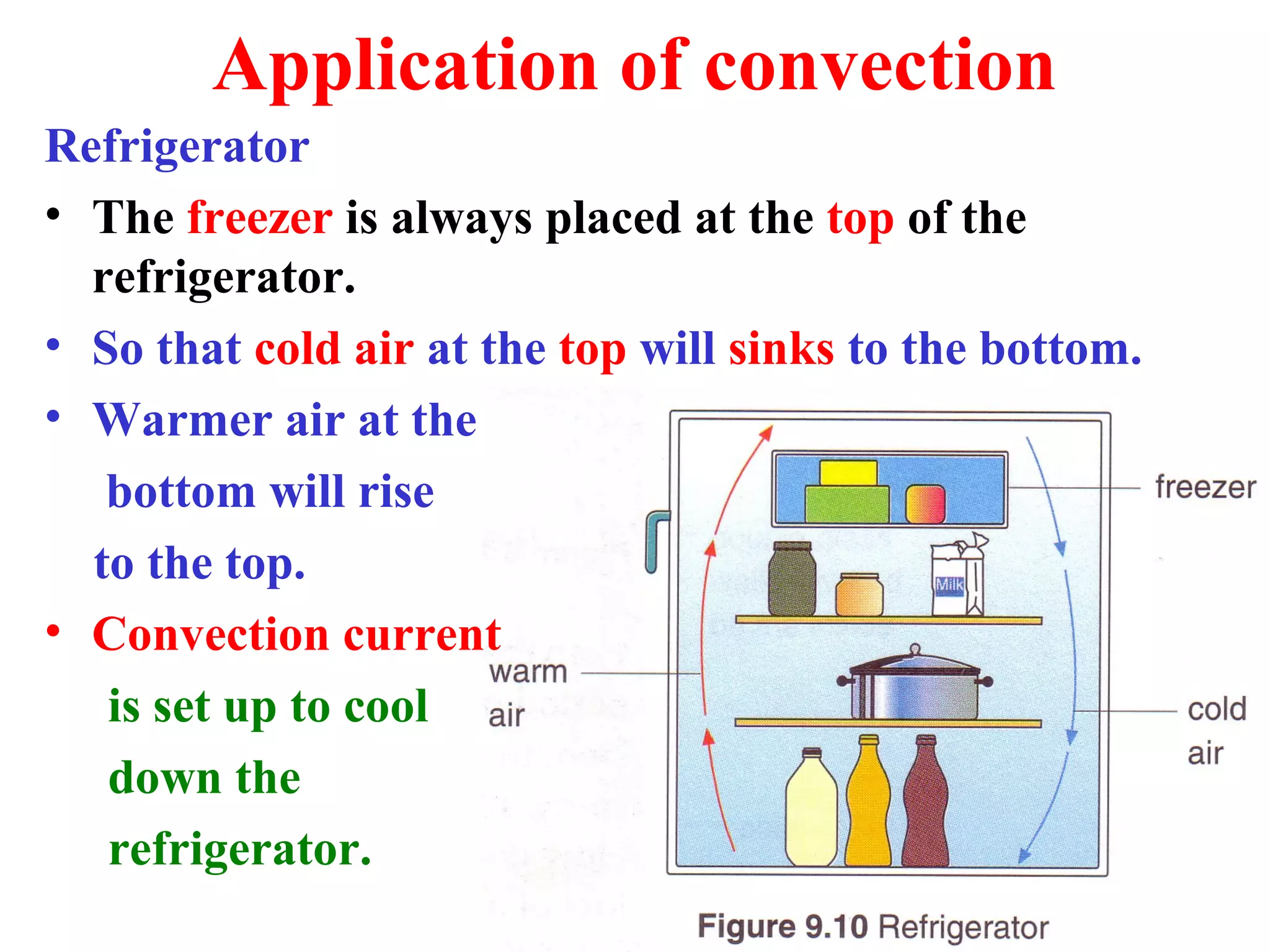
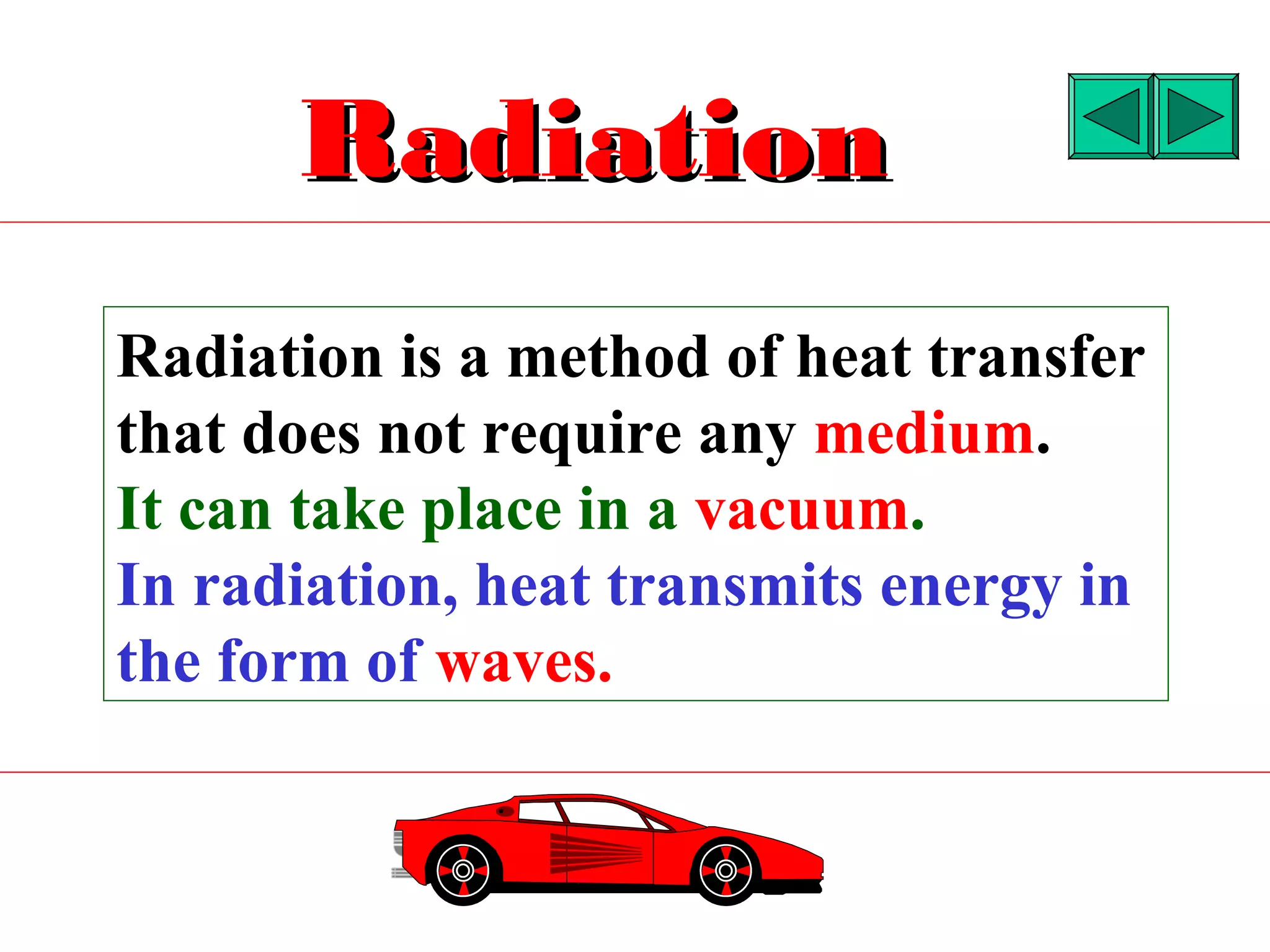
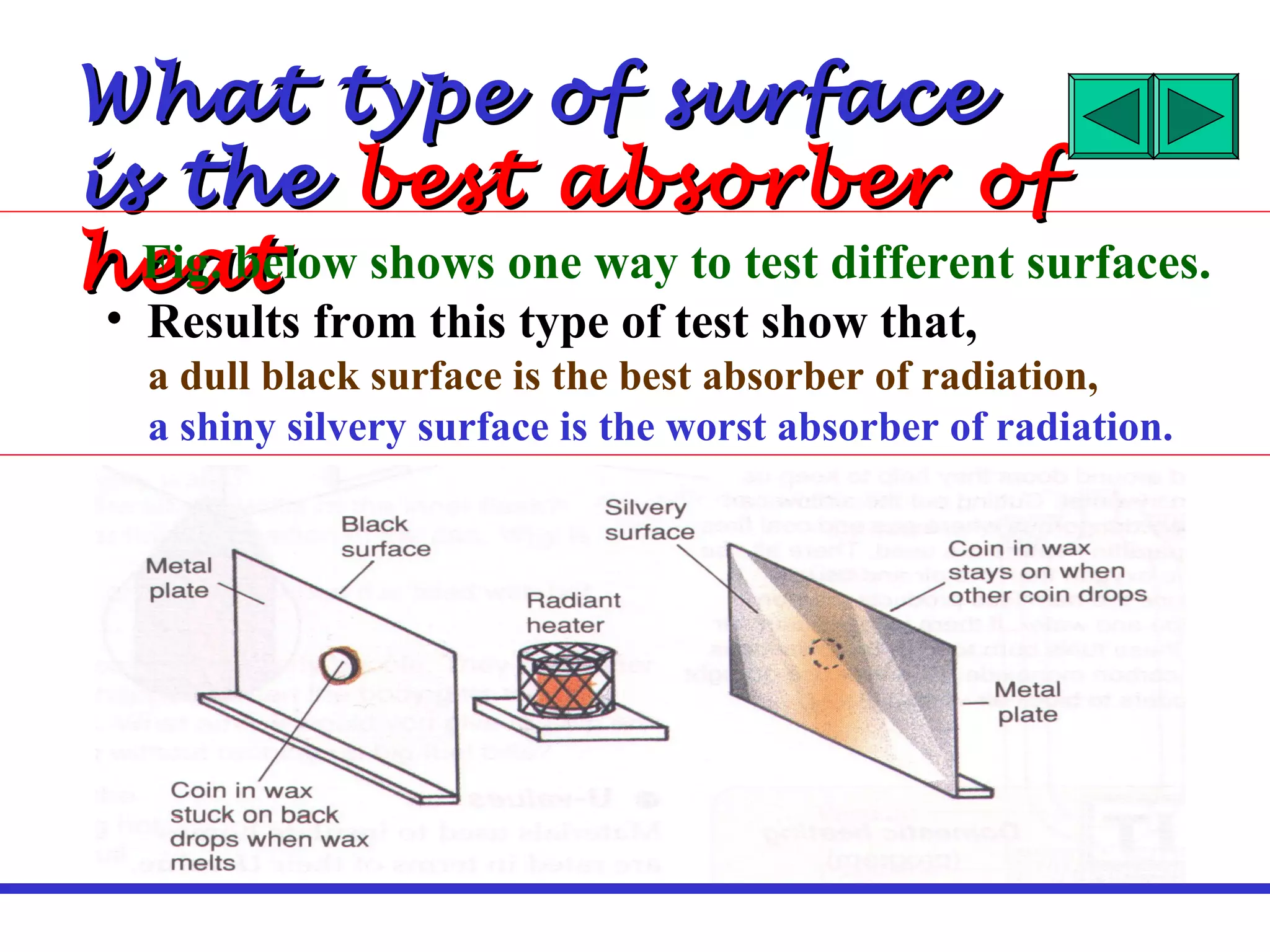
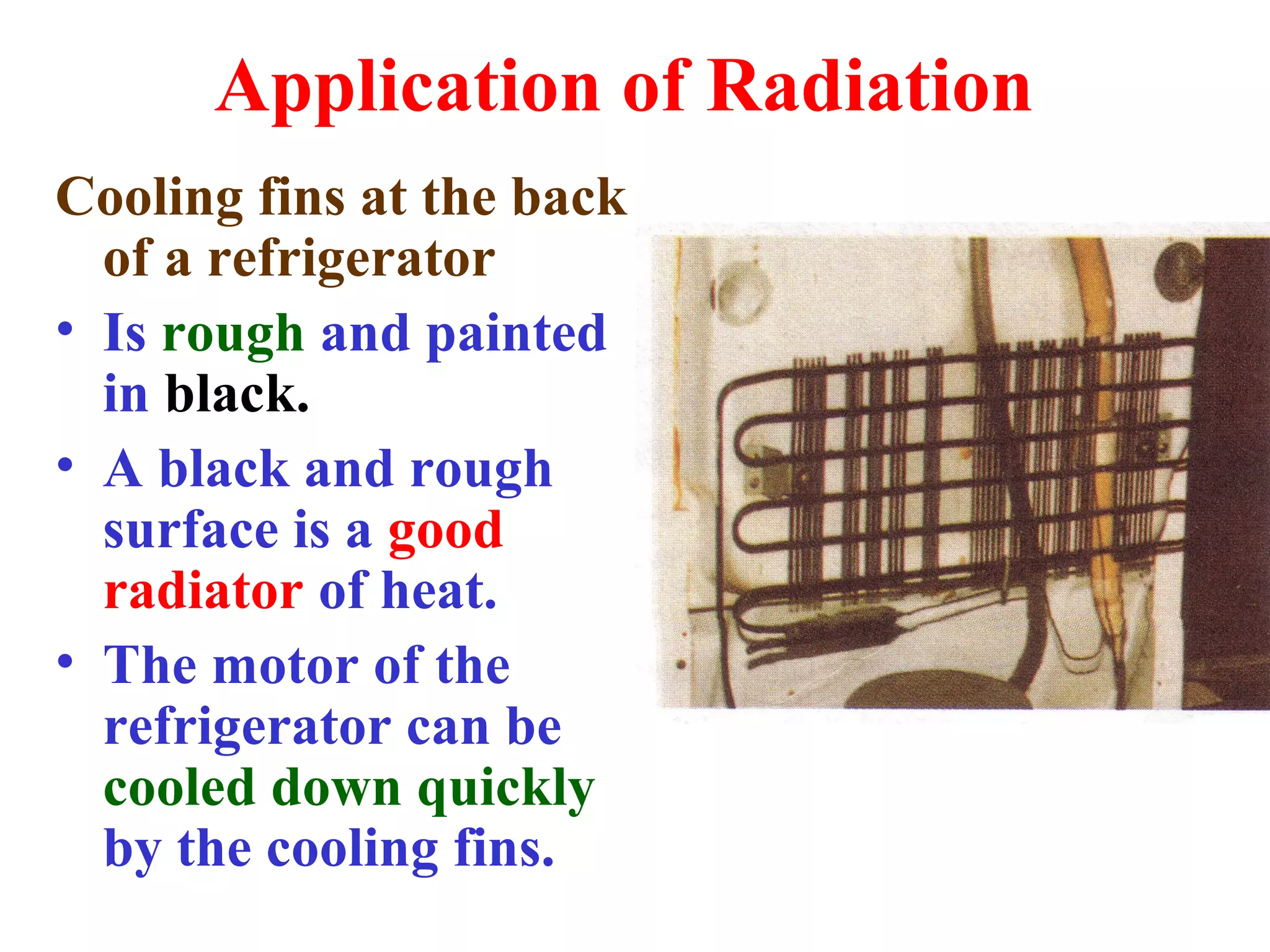
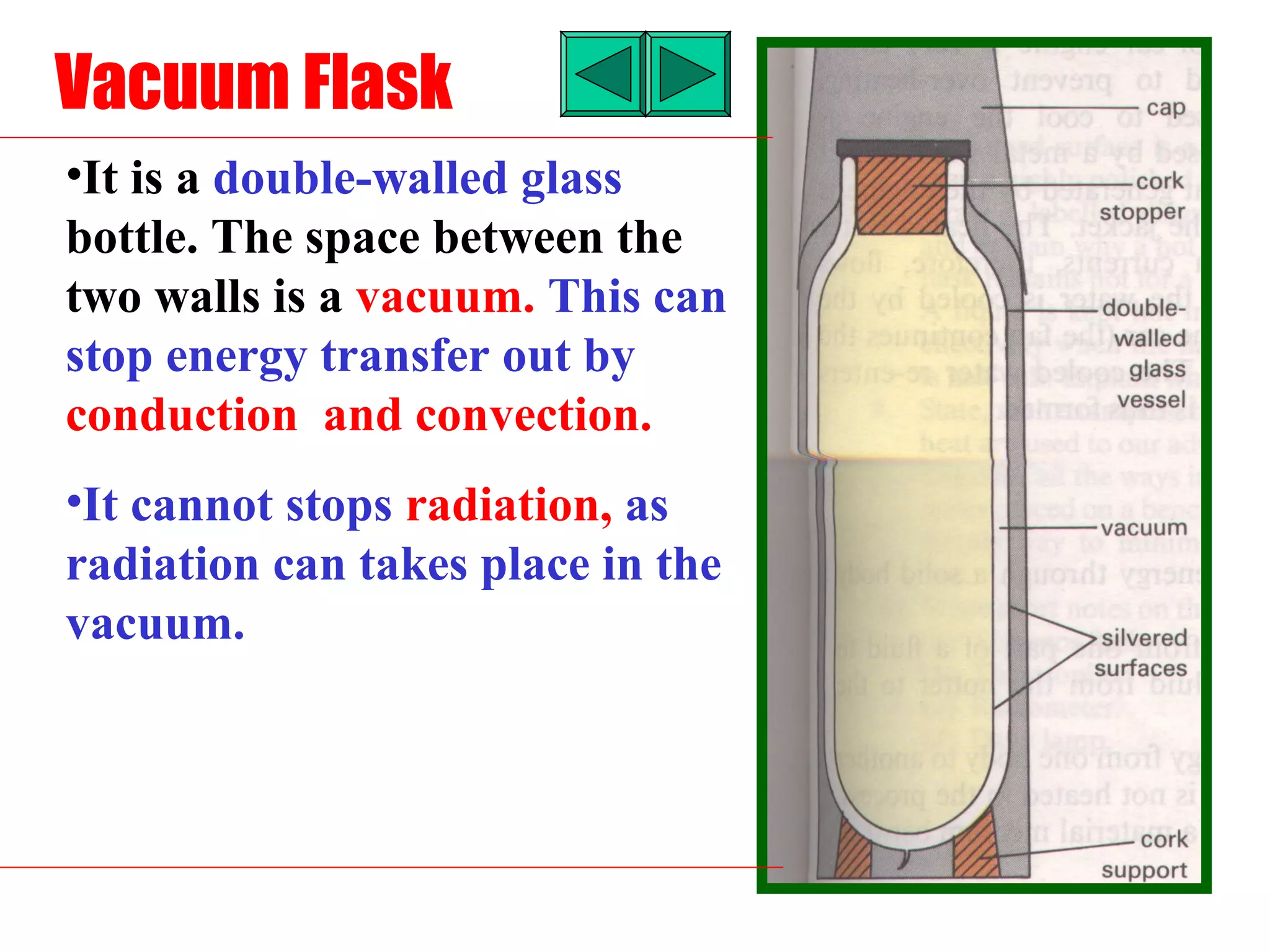
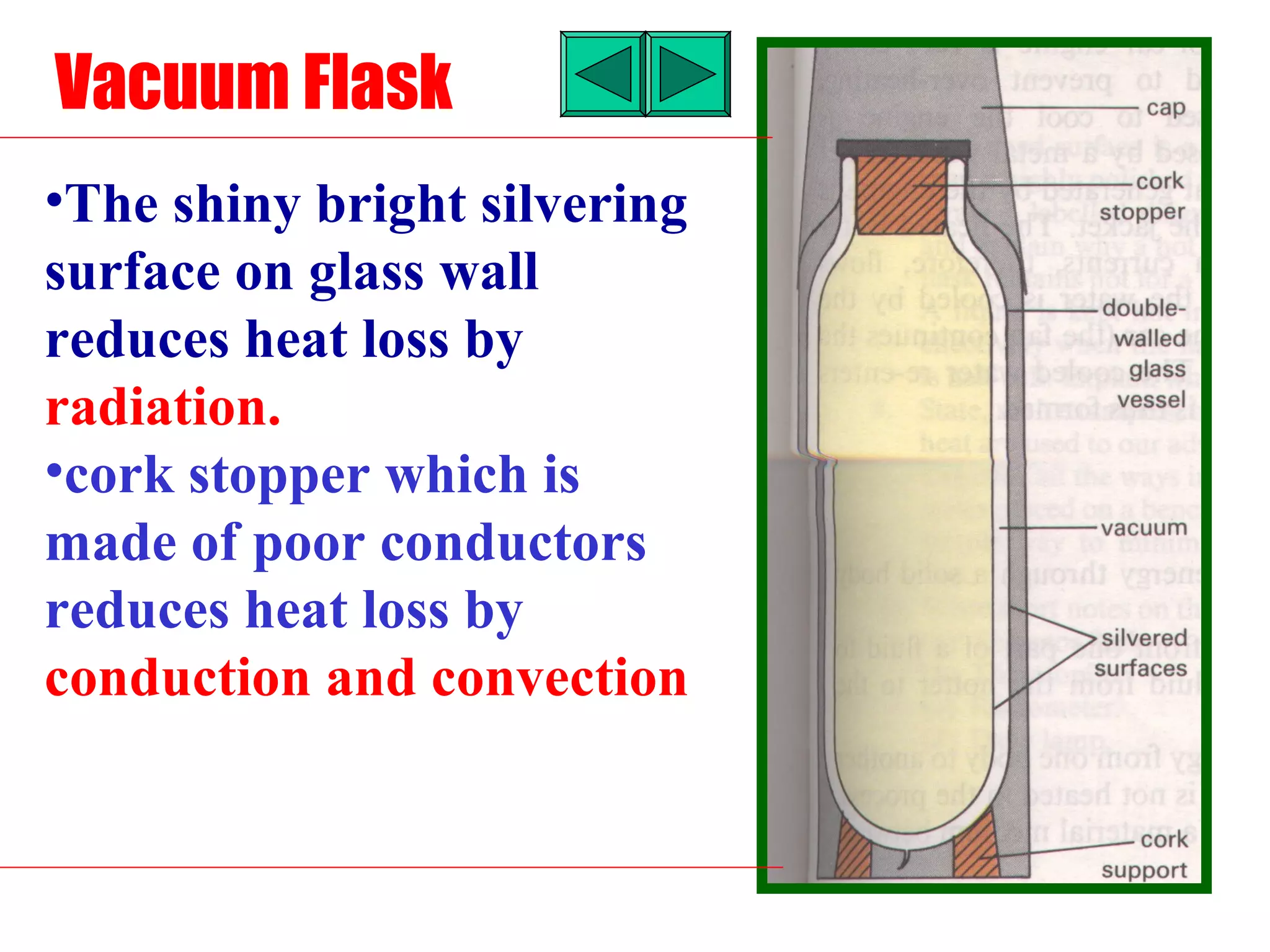
Heat can be transferred through three methods: conduction, convection, and radiation. Conduction involves the transfer of heat between objects in direct contact through vibrations of atoms. Convection involves the transfer of heat by the circulation of fluids like air and water. Radiation involves the transfer of heat through electromagnetic waves and does not require a medium. Each method allows for heat to travel from warmer to cooler regions until thermal equilibrium is reached.