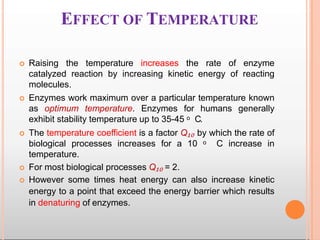
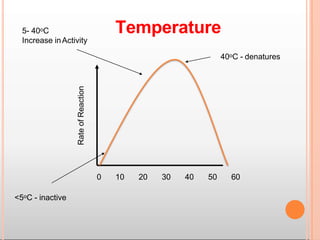
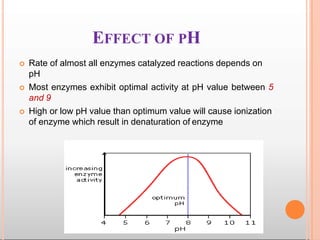
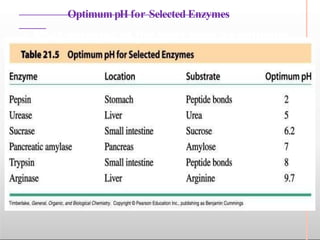
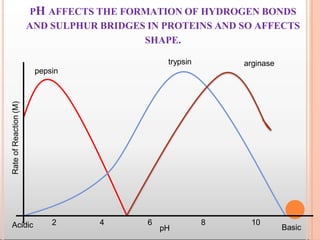
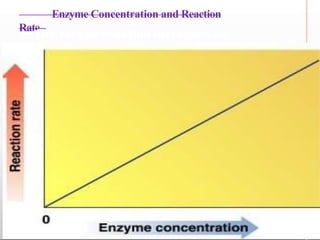
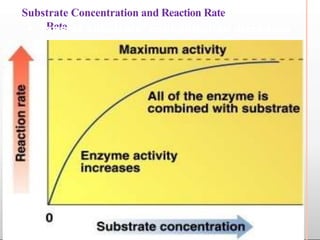
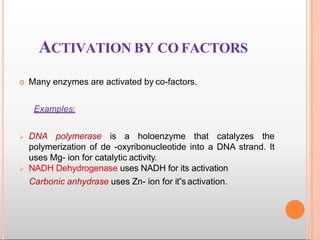
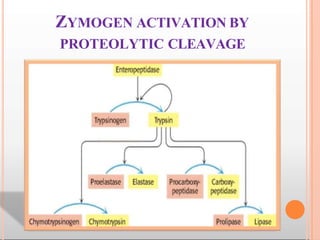
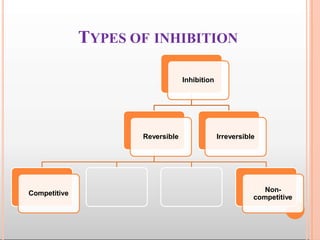
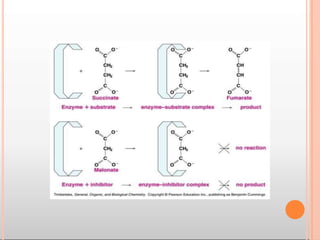
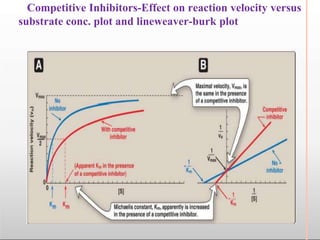
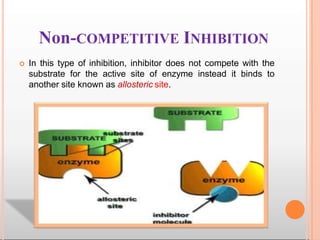
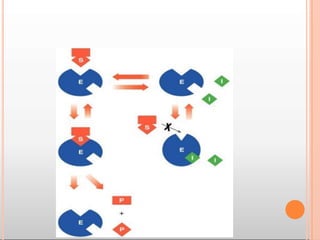
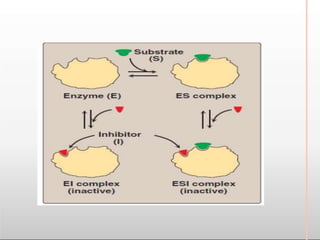
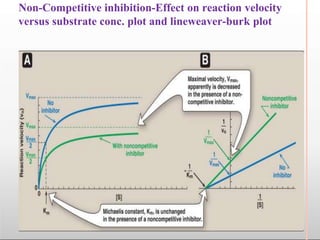
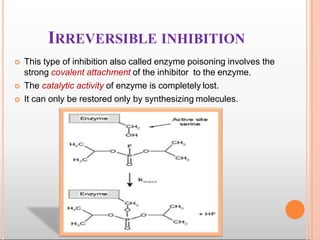
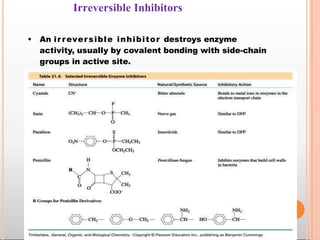
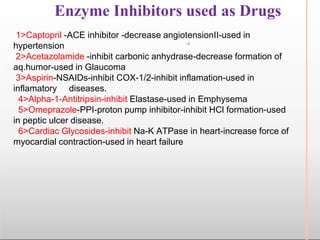
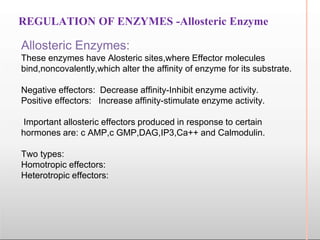
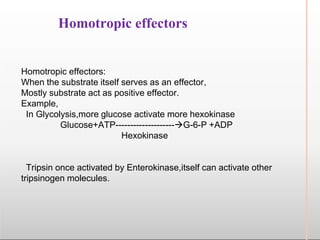
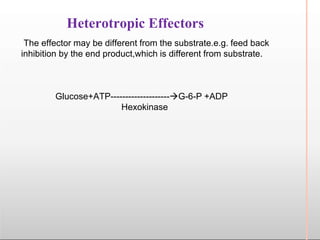
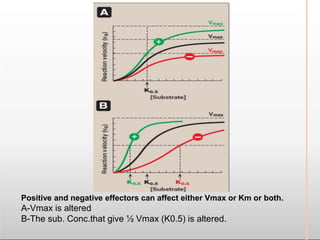
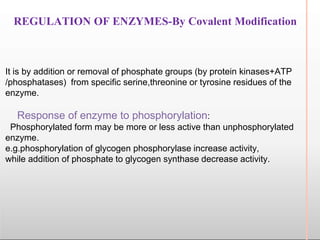
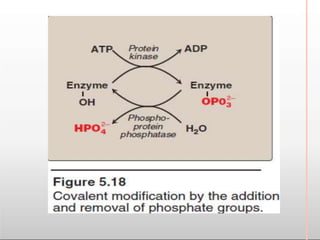
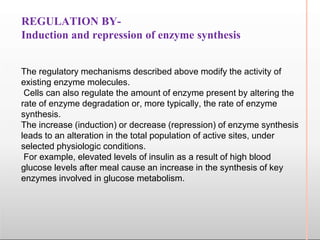
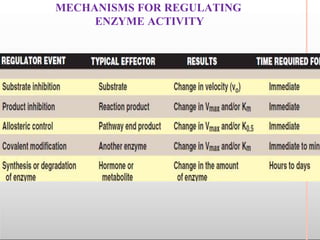
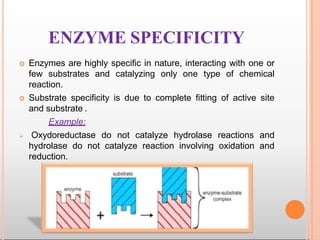
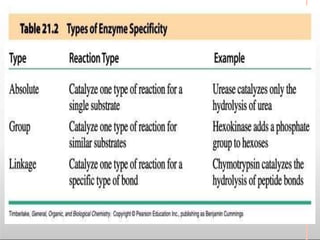
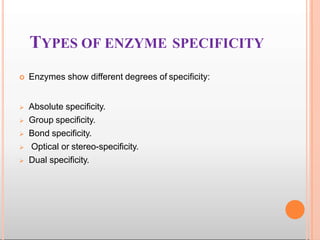
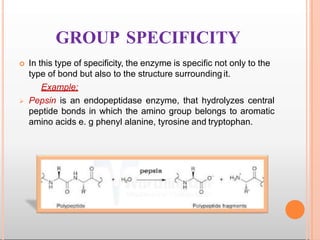
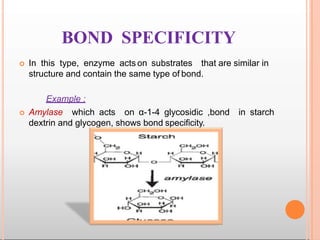
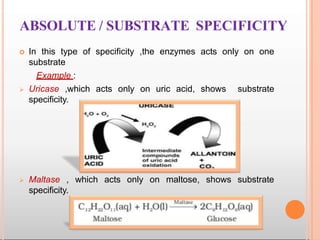
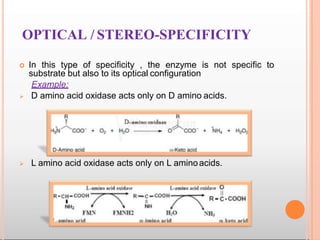
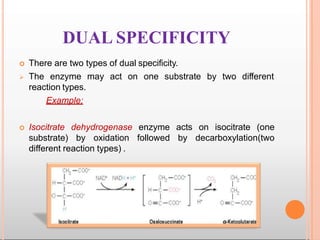
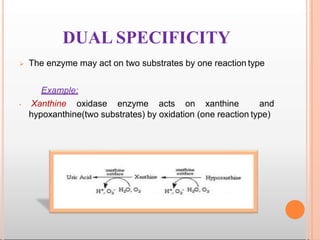
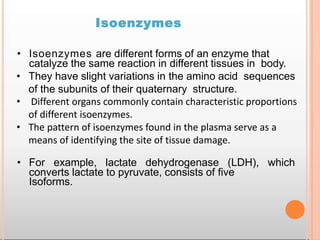
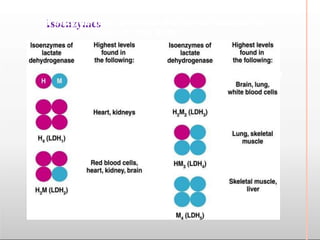
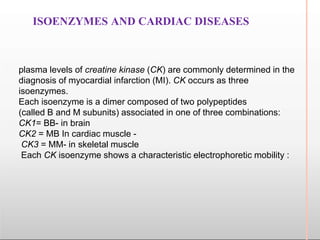
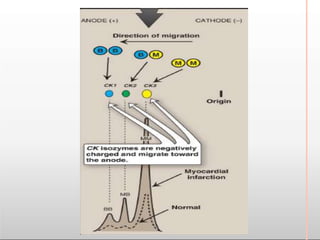
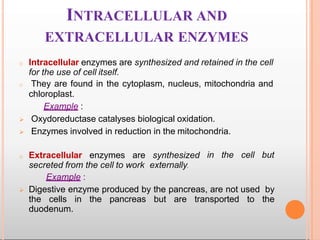
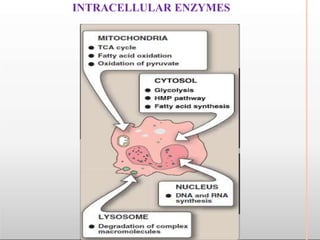
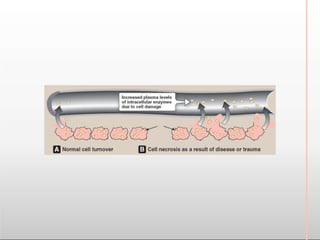
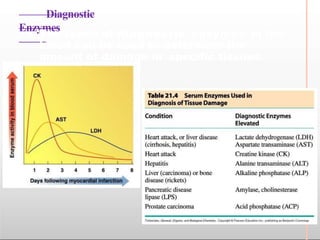
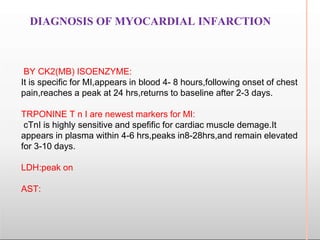
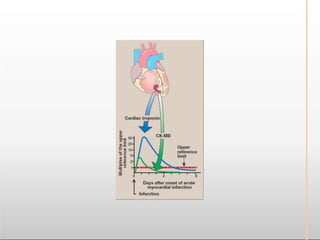
Environmental factors such as temperature, pH, and concentrations of enzymes, substrates, and products can affect the rate of enzyme-catalyzed reactions. Enzyme activity is also regulated by cofactors, allosteric effectors, covalent modification, and induction/repression of enzyme synthesis. Enzymes exhibit varying degrees of specificity, from absolute specificity for a single substrate to bond or group specificity for classes of substrates. Isoenzymes are variants of the same enzyme found in different tissues.