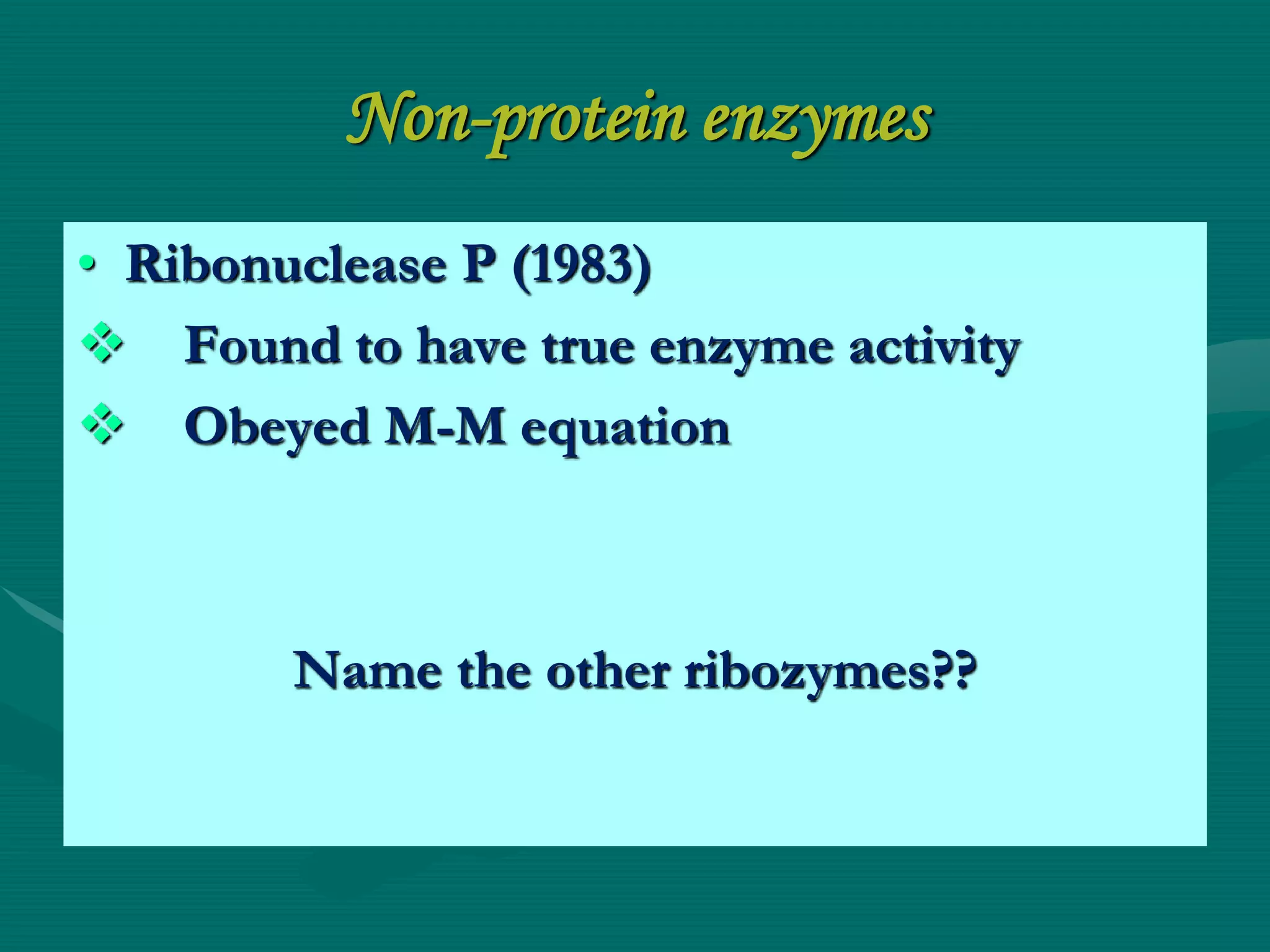
1. Enzymes can be inhibited through various mechanisms including competitive, non-competitive, uncompetitive, and allosteric inhibition. Competitive inhibitors compete with the substrate for the active site, increasing Km and leaving Vmax unchanged. Non-competitive inhibitors bind elsewhere, decreasing Vmax but not affecting Km.
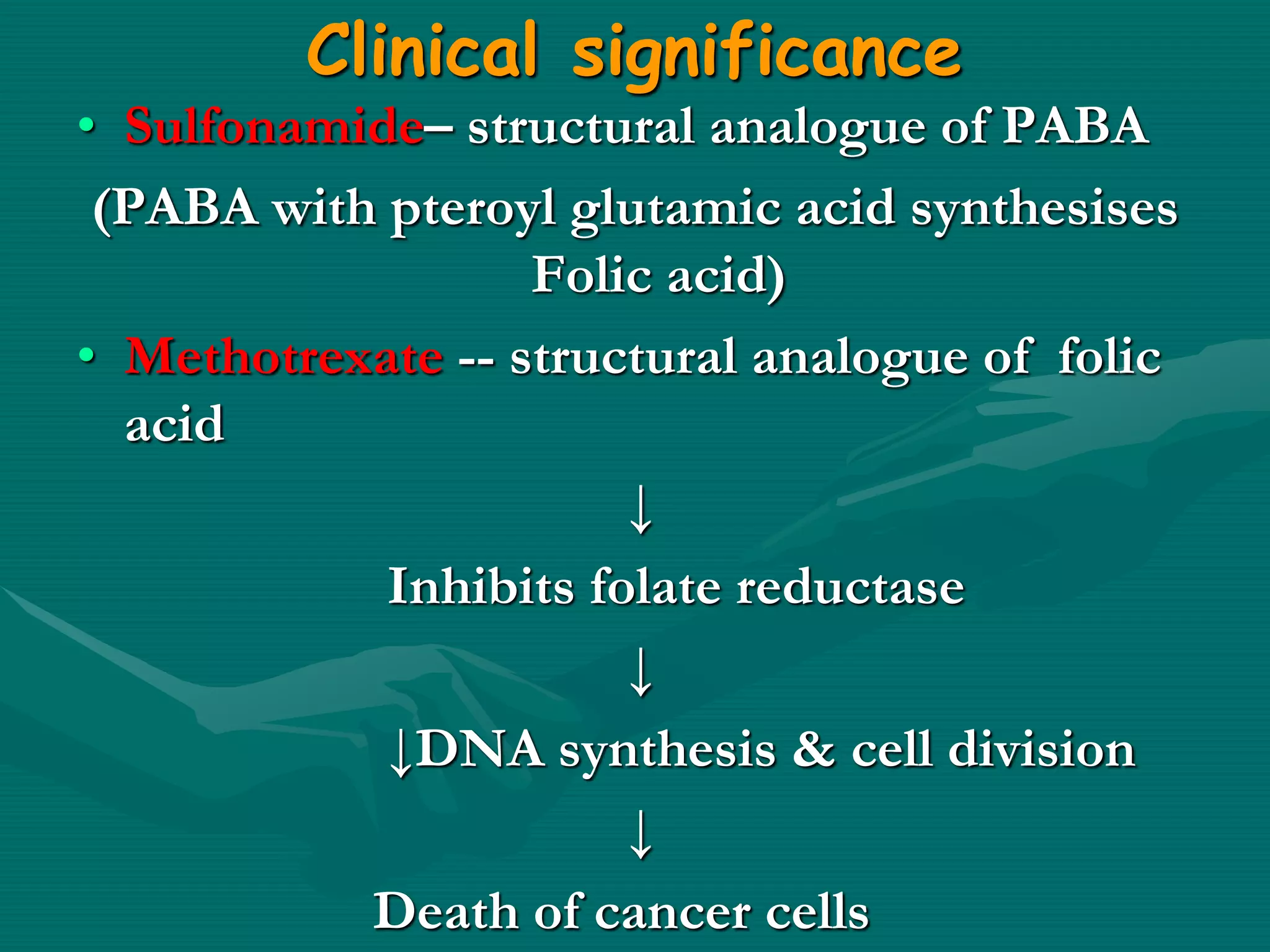
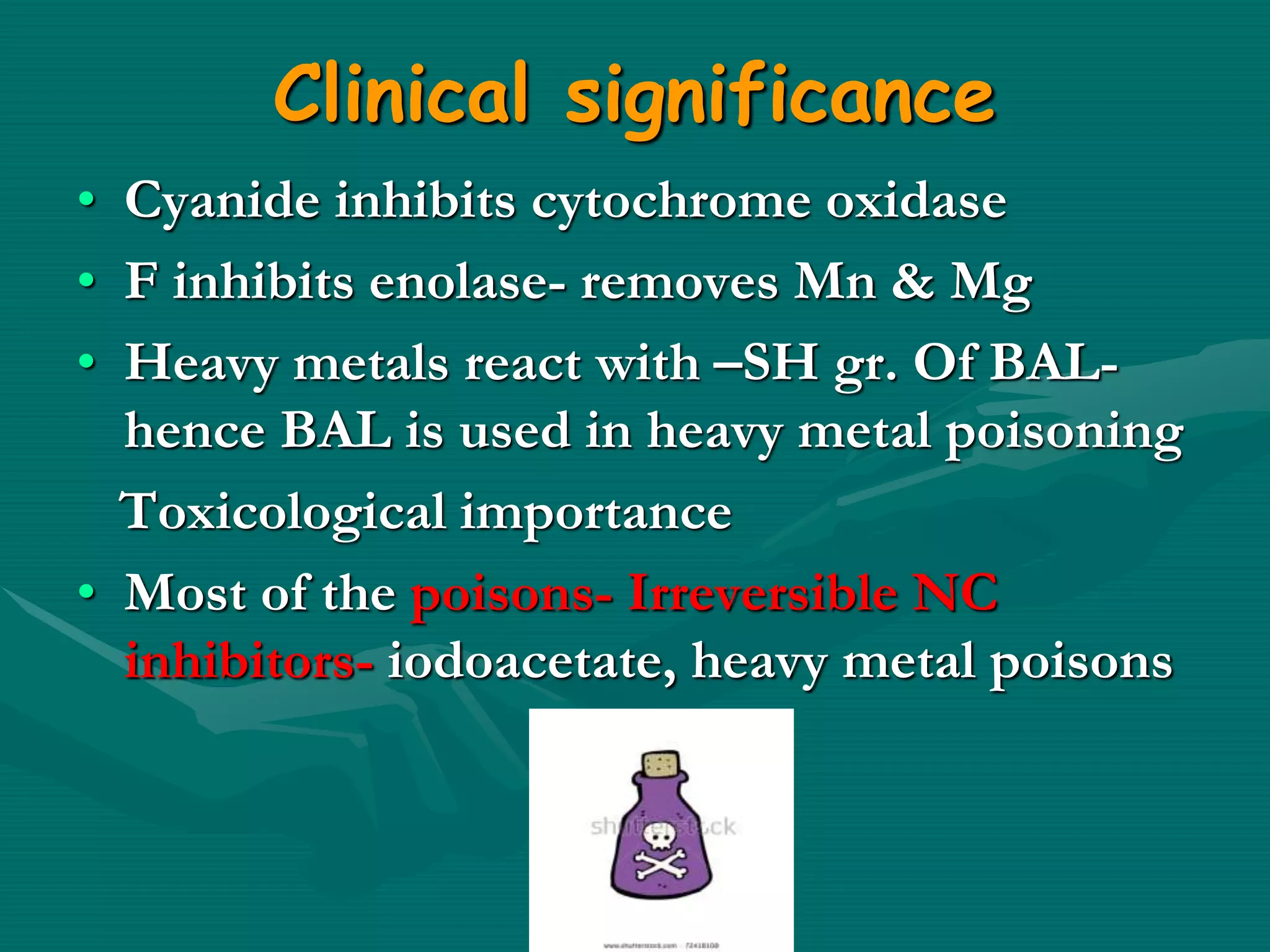
2. Enzyme inhibition has clinical significance in drug action and toxicology. Competitive inhibitors like sulfonamides and methotrexate are used as drugs, while non-competitive inhibitors like cyanide and heavy metals can be toxic.
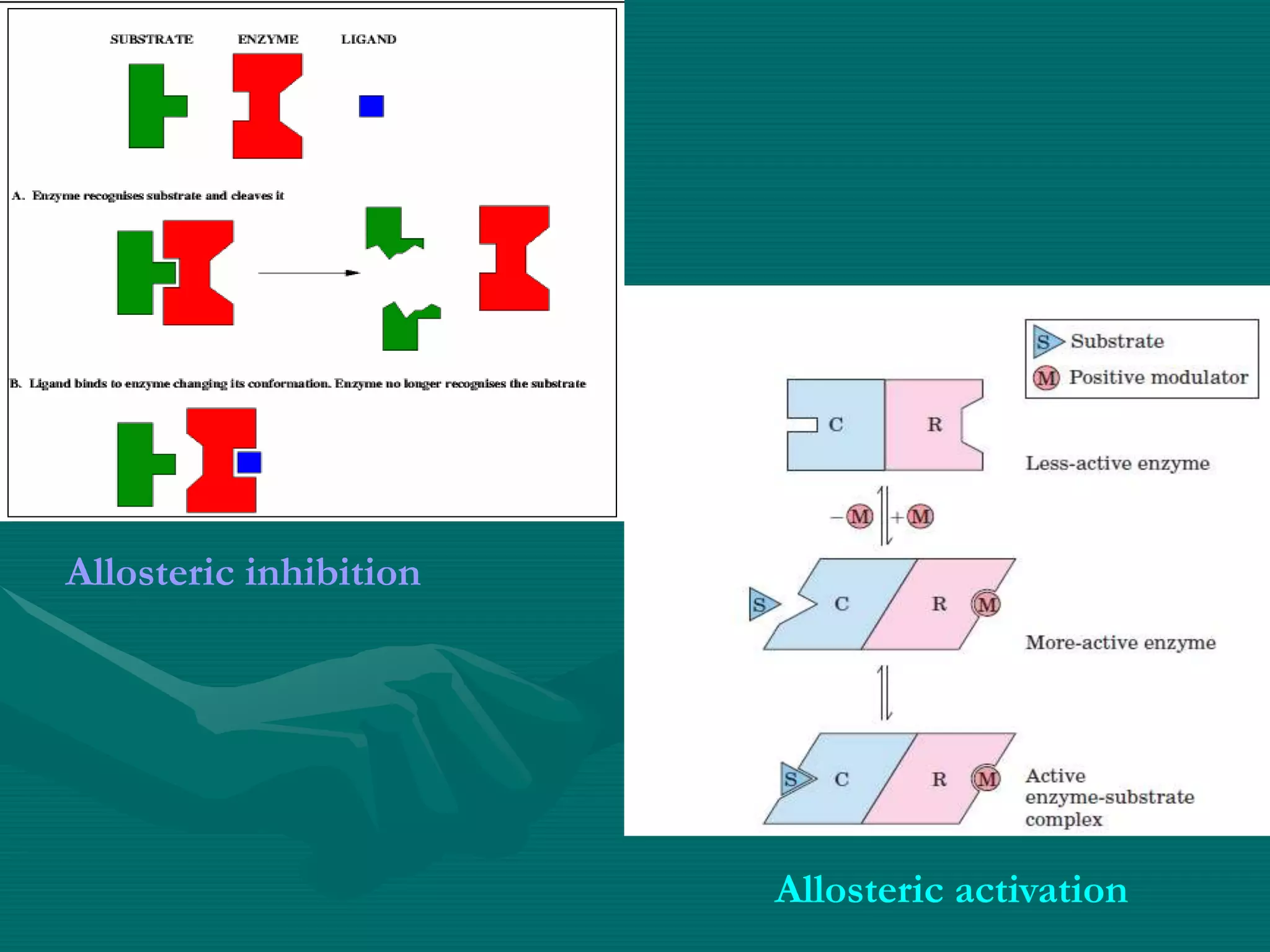
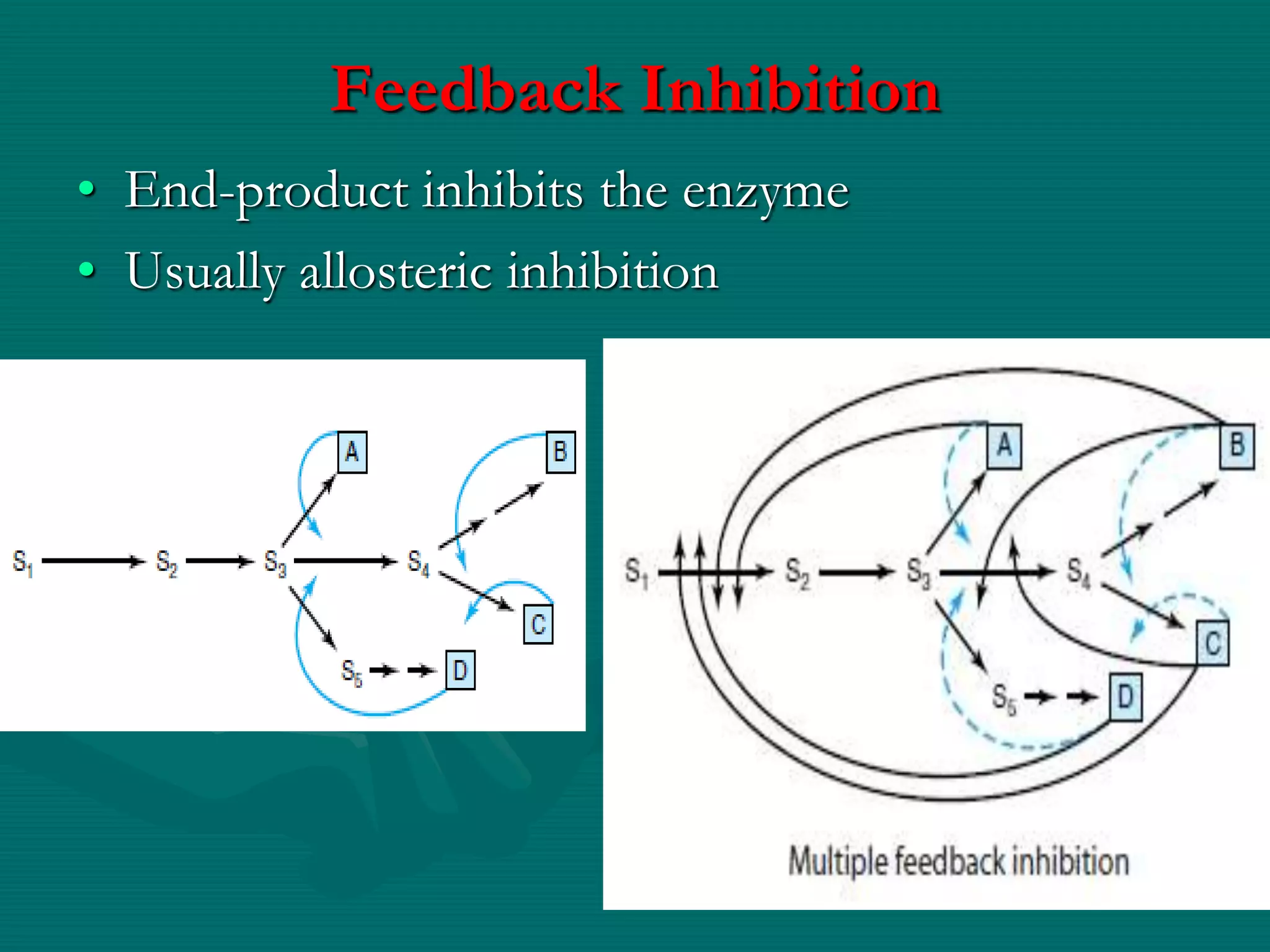
3. Allosteric inhibition involves effectors binding at sites other than the active site to induce a conformational change, decreasing Vmax




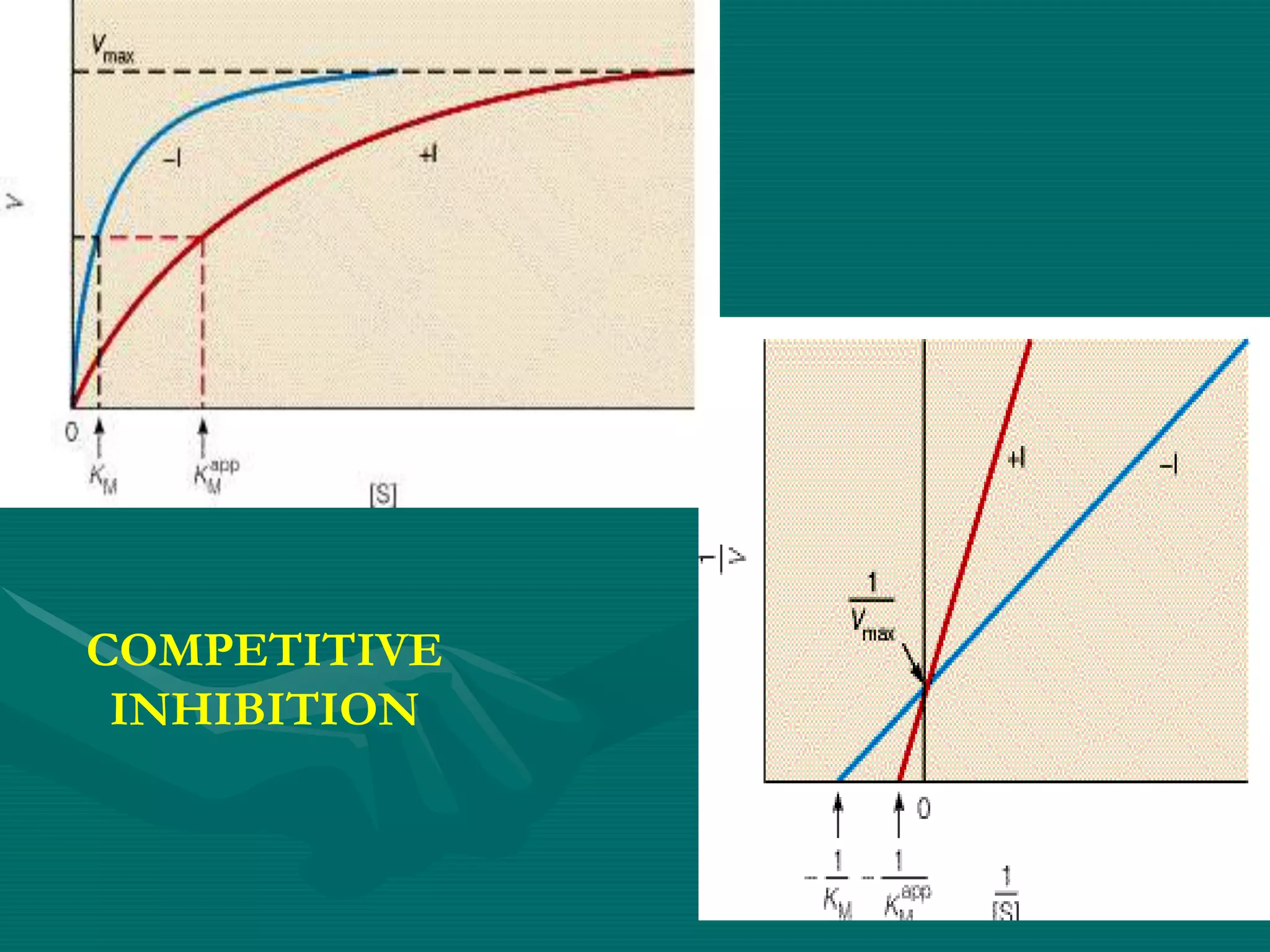
![Competitive inhibition
• Inhibitor competes with the substrate for the active site
• Inhibitor is substrate analogue
• Usually reversible
• ↑ [S] abolishes inhibition
• ↓ velocity of reaction
• ↑ Km
• Vmax unchanged](https://image.slidesharecdn.com/5enzymeinhibition-171108093403/75/5-enzyme-inhibition-5-2048.jpg)


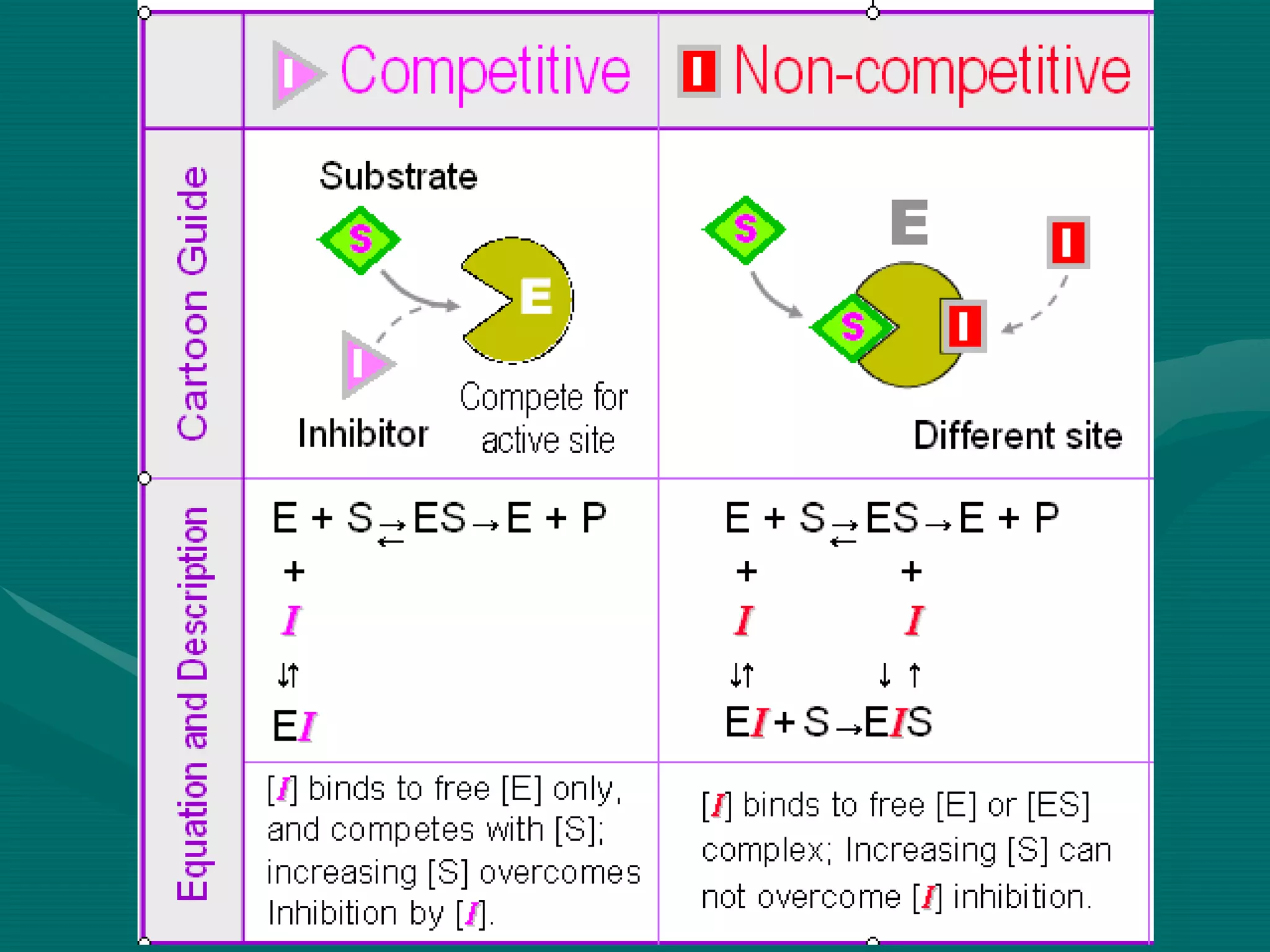
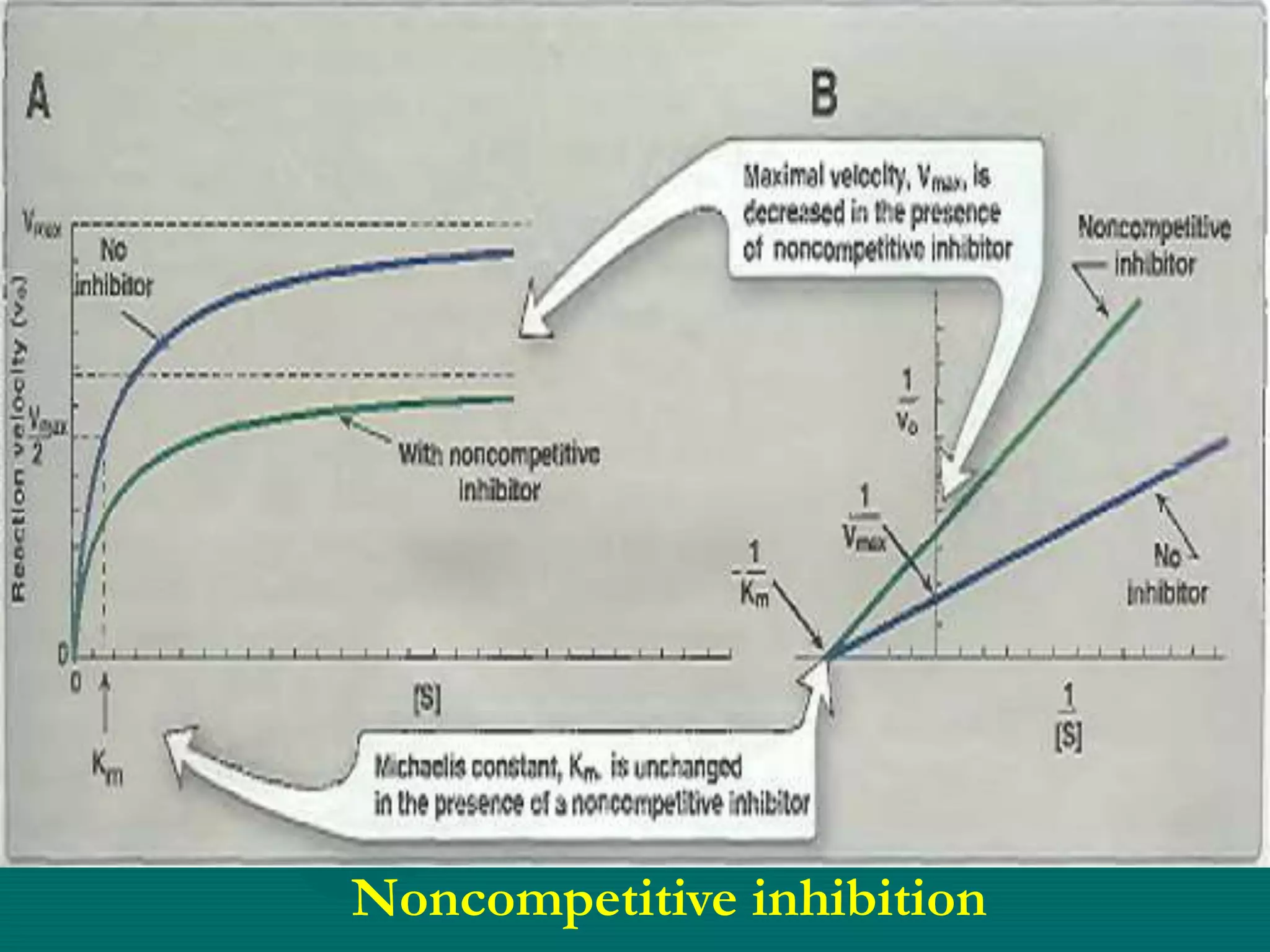
![Non-competitive inhibition
• No competition between substrate and
inhibitor
• Different binding sites
• No structural similarities
• ↑ [S] doesn’t resolve the inhibition
• Usually irreversible
• May be reversible when inhibitor is
removed
• Km value unchanged
• Vmax reduces](https://image.slidesharecdn.com/5enzymeinhibition-171108093403/75/5-enzyme-inhibition-10-2048.jpg)


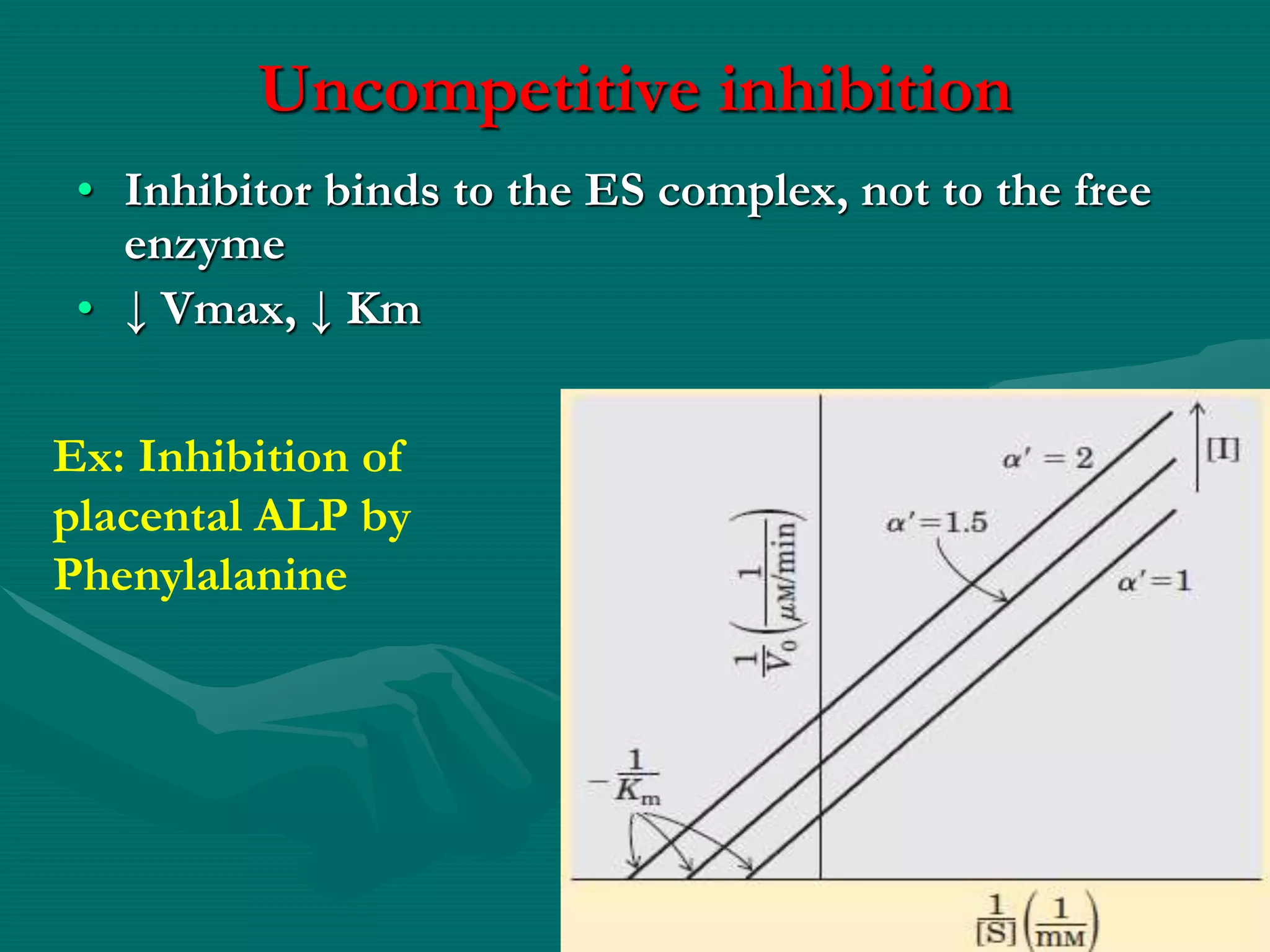



![• Partially reversible; excess substrate
may reverse the reaction
• ↑ Km
• ↓Vmax
• Effect of modifier is maximum when
[s]≈ Km](https://image.slidesharecdn.com/5enzymeinhibition-171108093403/75/5-enzyme-inhibition-21-2048.jpg)