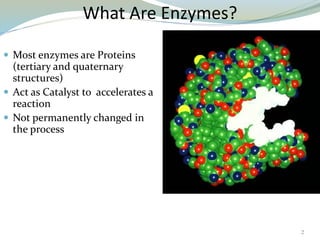
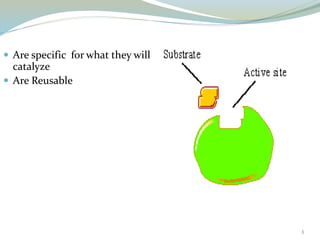
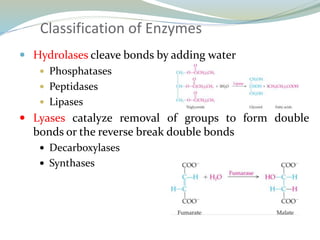
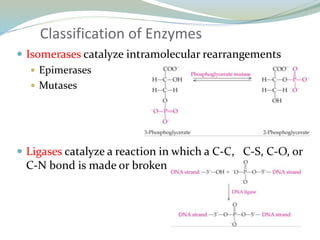
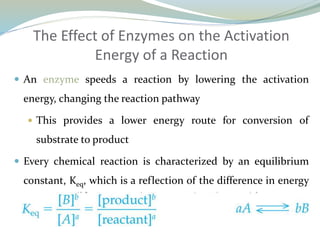
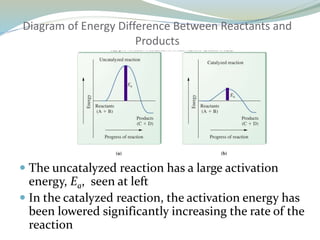
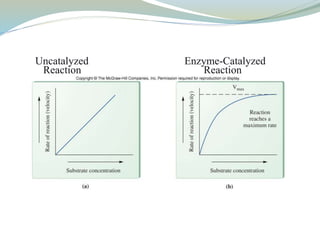
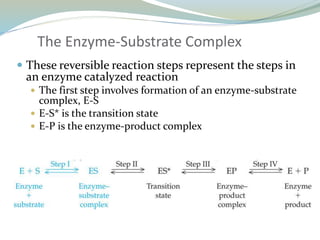
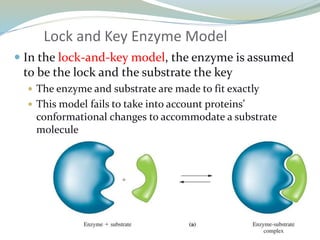
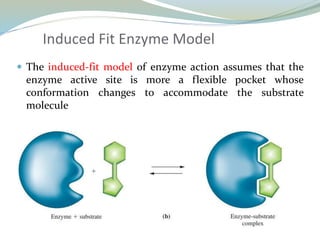
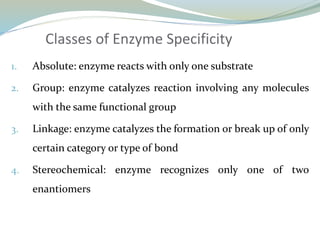
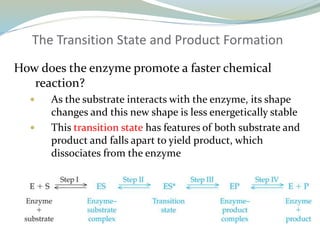
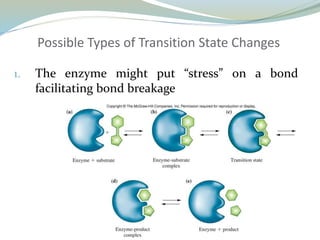
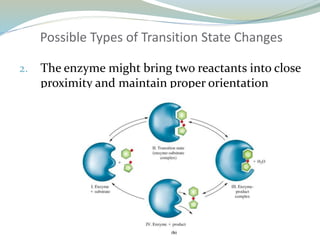
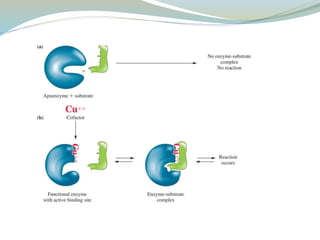
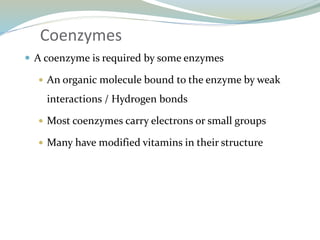
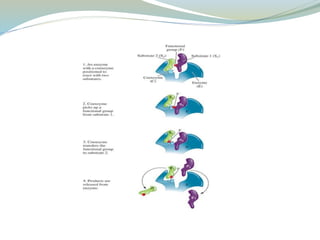
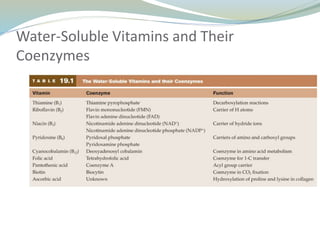
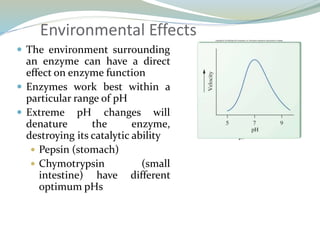
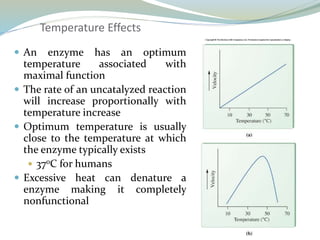
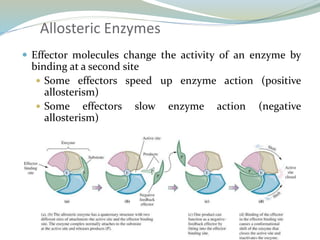
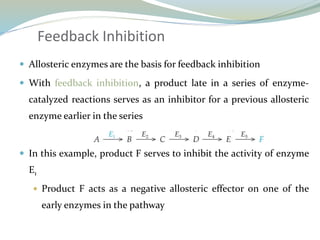
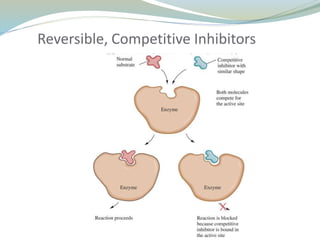
Enzymes are protein catalysts that lower the activation energy of biochemical reactions without being consumed. They achieve specificity by fitting substrates into their active sites. Enzyme activity is regulated through various mechanisms including feedback inhibition, cofactors, pH, temperature, and allosteric regulation. Inhibitors bind enzymes to reduce their activity, either reversibly or irreversibly. Enzymes have many applications in medicine including diagnostics, analytical tests, and enzyme replacement therapy.