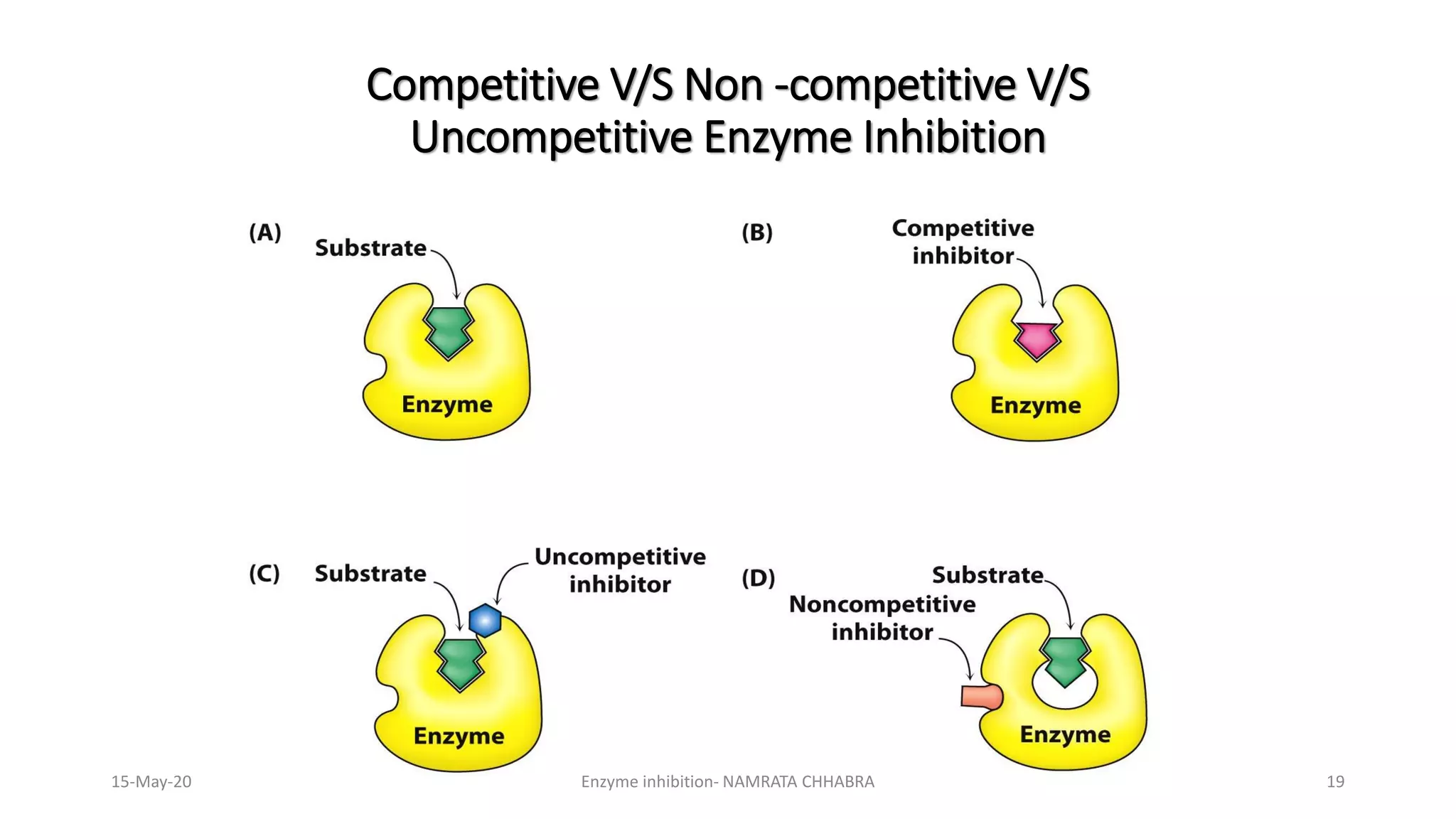
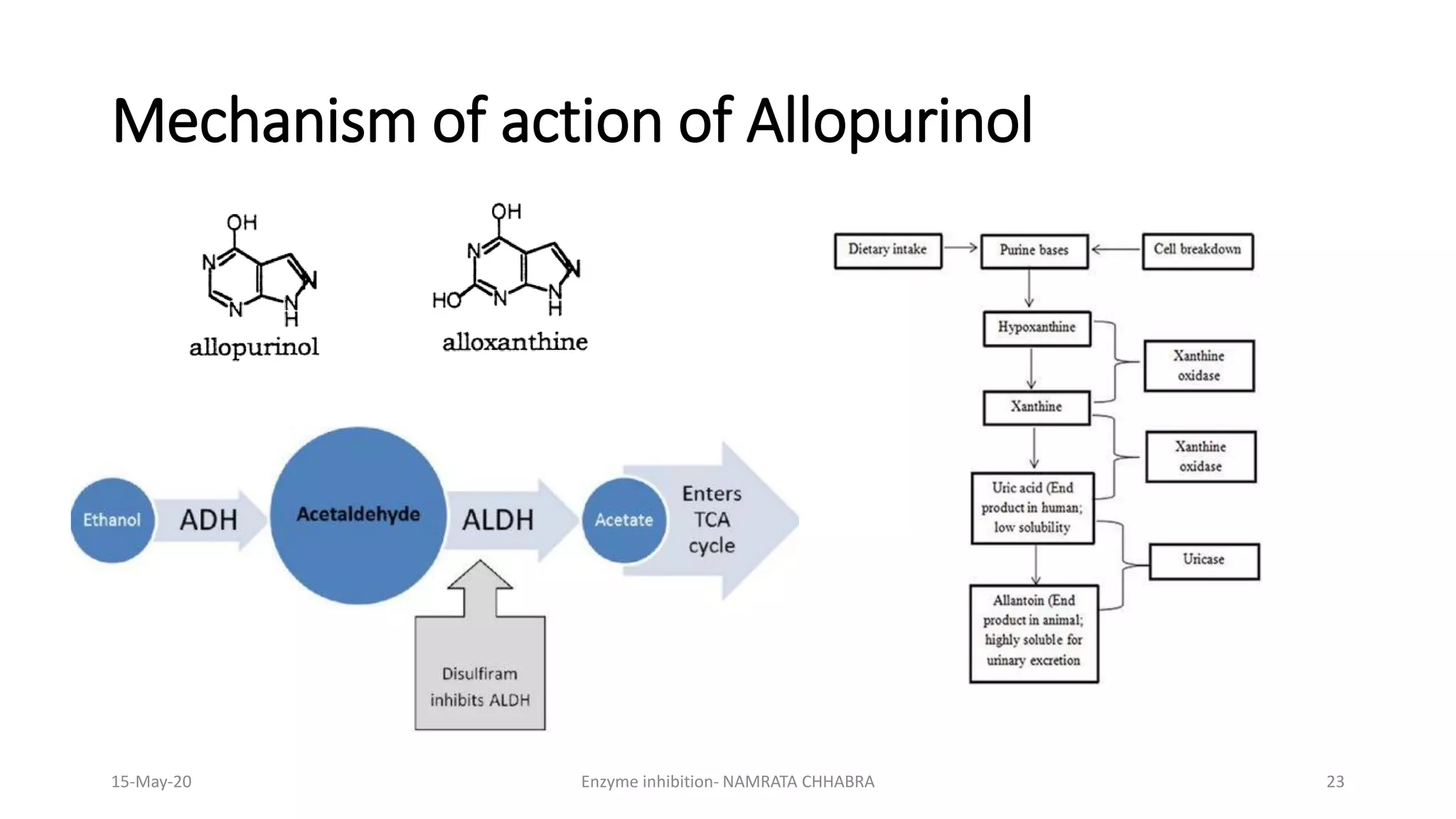
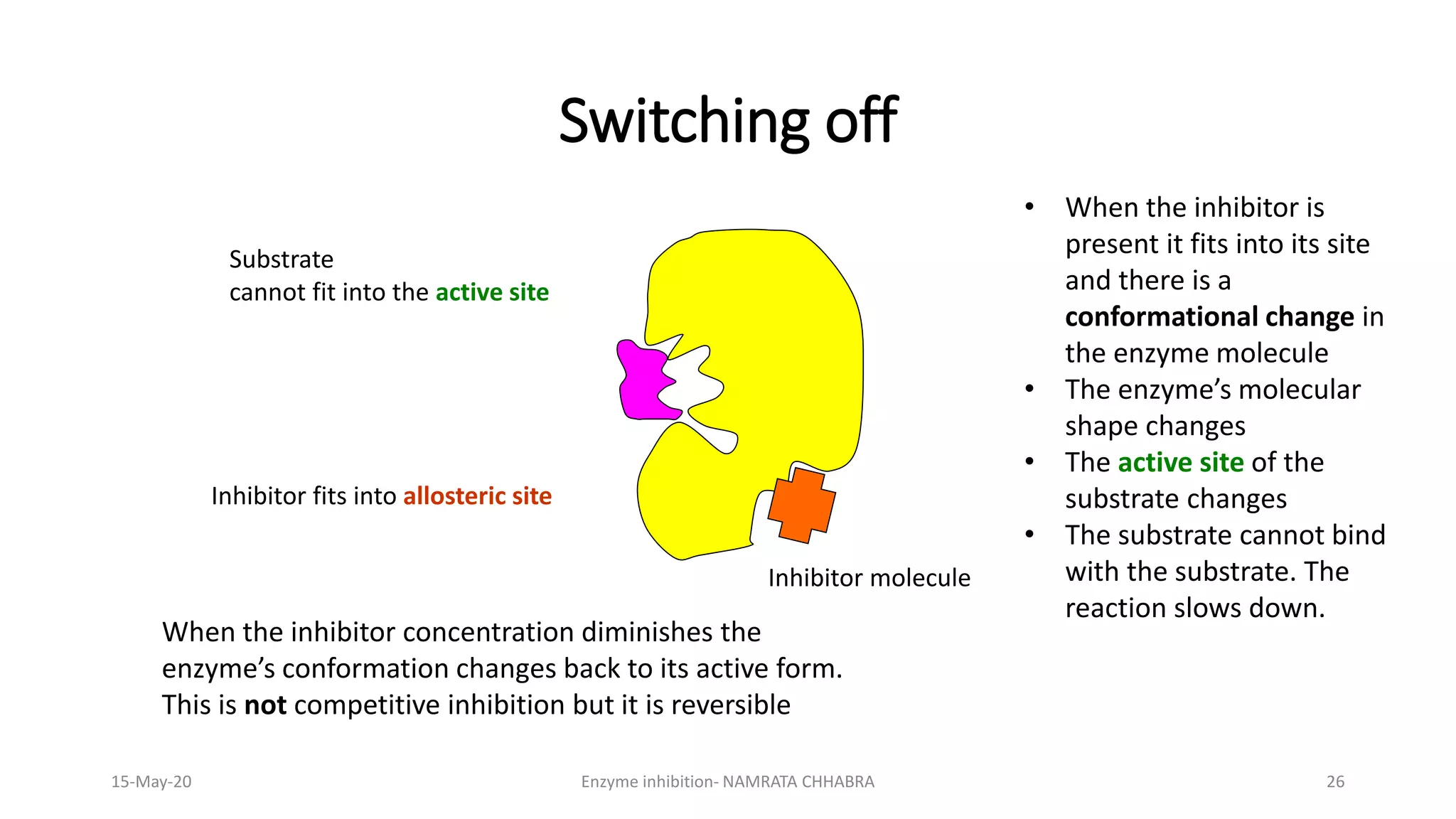
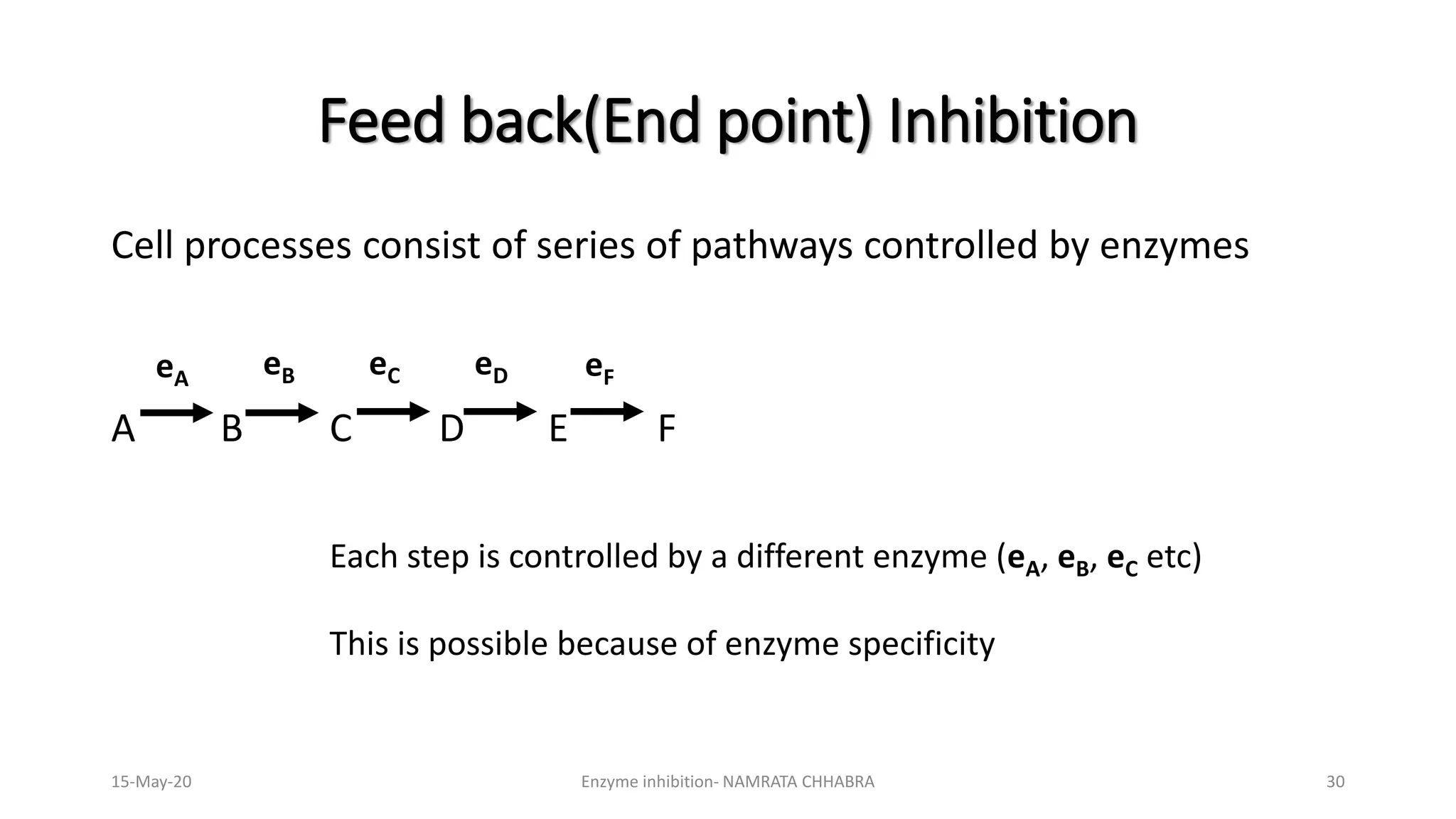
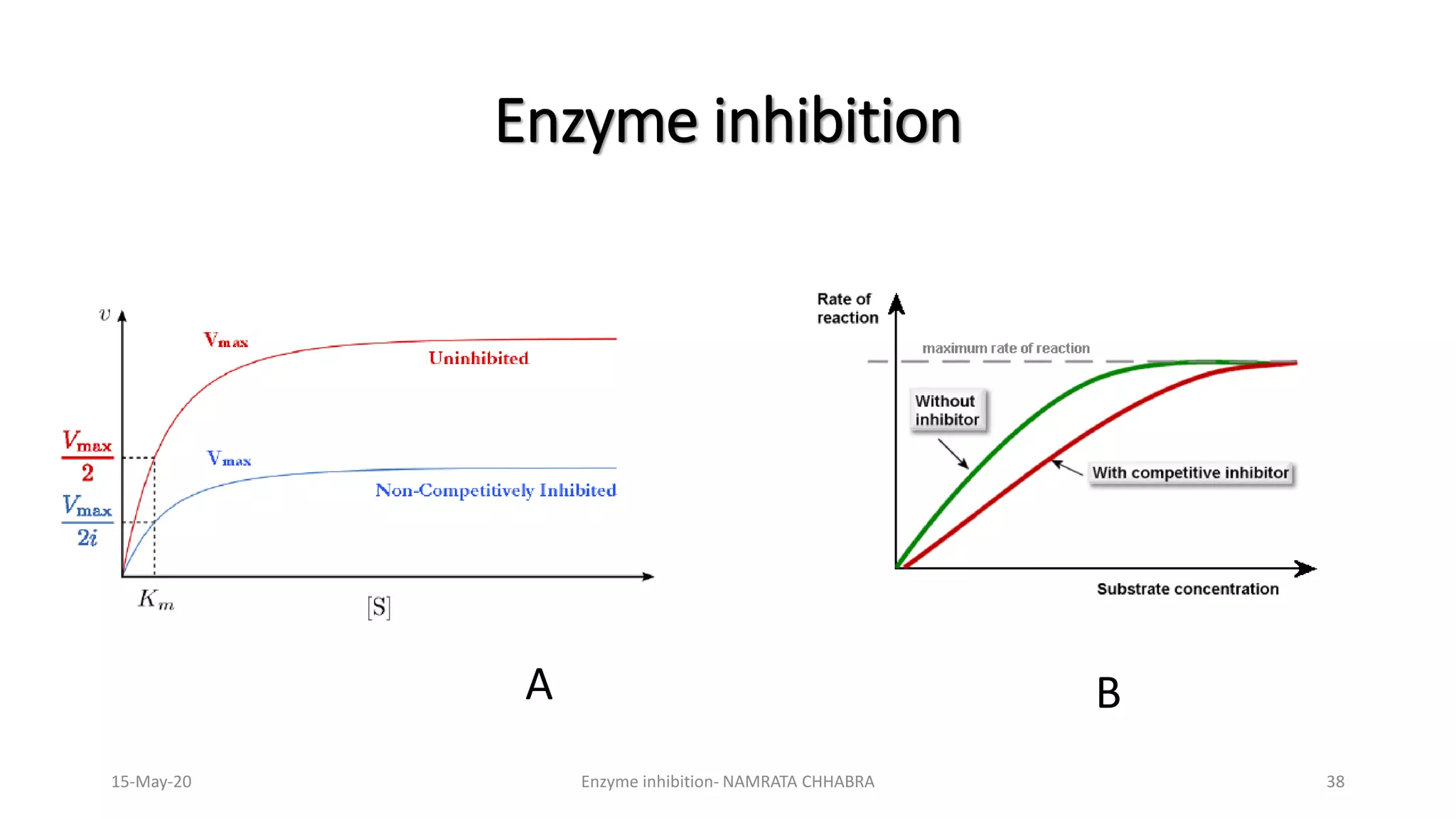
The document provides a comprehensive overview of enzyme inhibition, defining it as the reduction in the rate of enzyme-catalyzed reactions. It categorizes inhibitors into types, including competitive, non-competitive, uncompetitive, suicidal, and allosteric inhibitors, detailing their mechanisms and examples. Additionally, the document discusses the role of enzyme inhibitors in pharmacology, physiology, and the impact on drug development and therapeutics.














![Lineweaver Burk plot
In the presence of a competitive inhibitor,
Vmax can still be reached if sufficient
substrate is available,
one-half Vmax requires a higher [S] than
before and thus Km is larger.
With noncompetitive inhibition, enzyme
rate (velocity) is reduced for all values of [S],
including
Vmax and one-half Vmax but
Km remains unchanged.
15-May-20 Enzyme inhibition- NAMRATA CHHABRA 16](https://image.slidesharecdn.com/enzymeinhibition-200515002439/75/Enzyme-inhibition-16-2048.jpg)