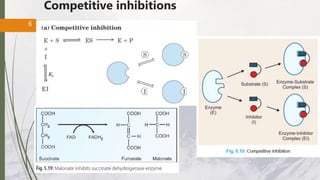
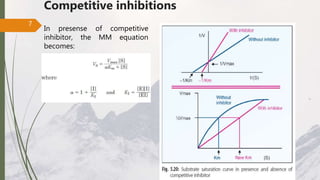
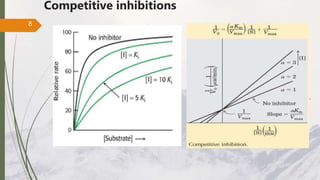
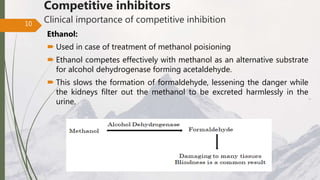
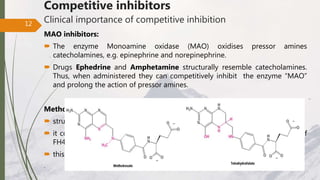
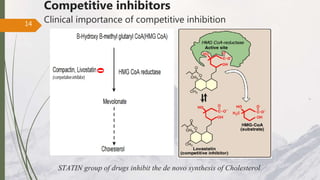
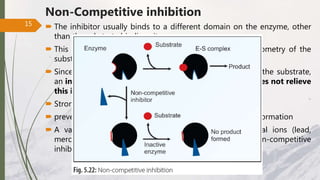
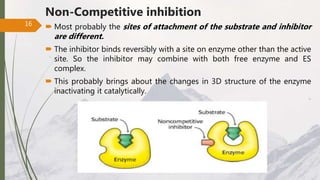
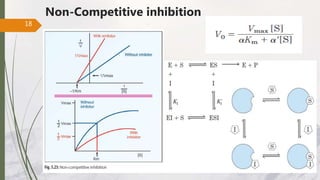
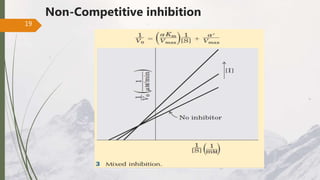
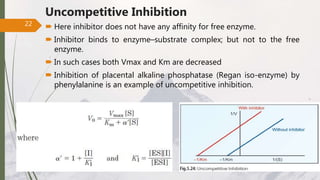
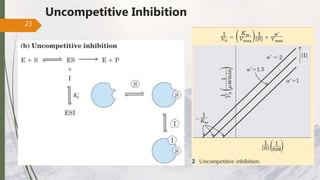
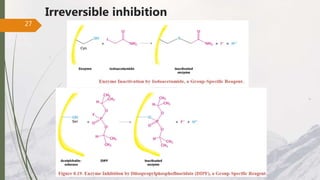
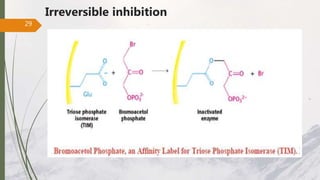
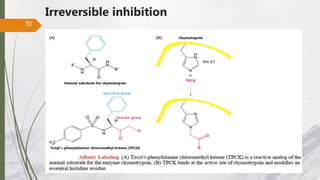
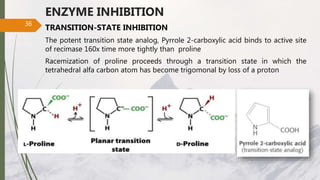
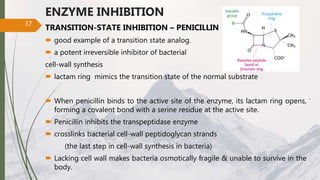
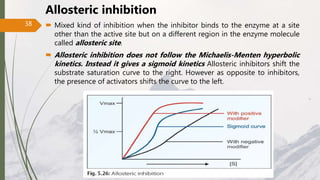
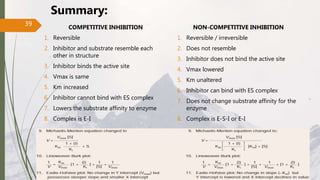
The document discusses different types of enzyme inhibition. There are three broad categories of enzyme inhibition: reversible, irreversible, and allosteric inhibition. Reversible inhibition includes competitive, non-competitive, and uncompetitive inhibition. Competitive inhibitors bind to the enzyme's active site, preventing substrate binding. Non-competitive inhibitors bind elsewhere, altering the enzyme's shape. Uncompetitive inhibitors only bind to the enzyme-substrate complex. Irreversible inhibitors covalently bind the enzyme, permanently inactivating it. Many drugs work by competitively or non-competitively inhibiting key enzymes.