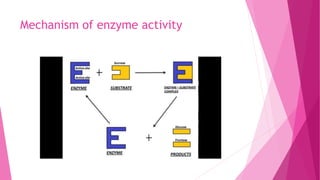
1. Enzymes are protein catalysts that regulate chemical reactions in living cells without undergoing any permanent changes themselves.
2. They function by binding to a specific substrate and lowering the activation energy of a reaction, greatly increasing the reaction rate.
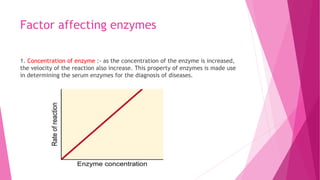
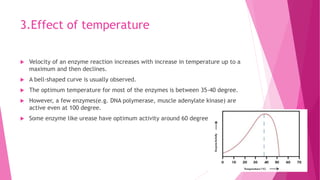
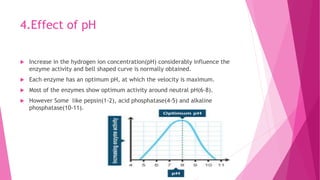
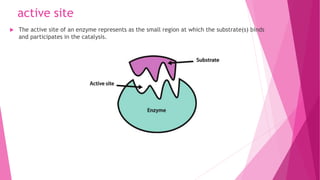
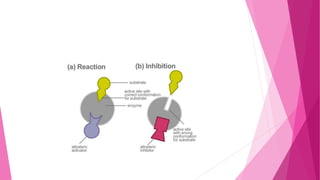
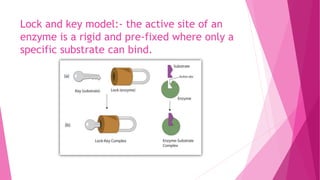
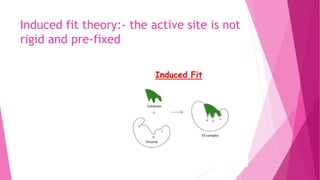
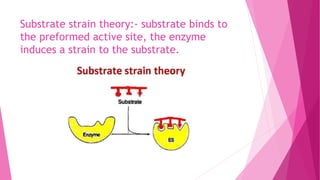
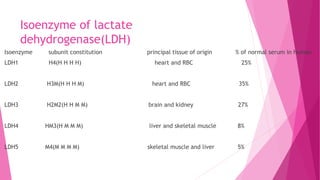
3. The active site of an enzyme is where substrate binding and catalysis occur. Factors like temperature, pH, and inhibitor binding can impact enzyme activity levels.