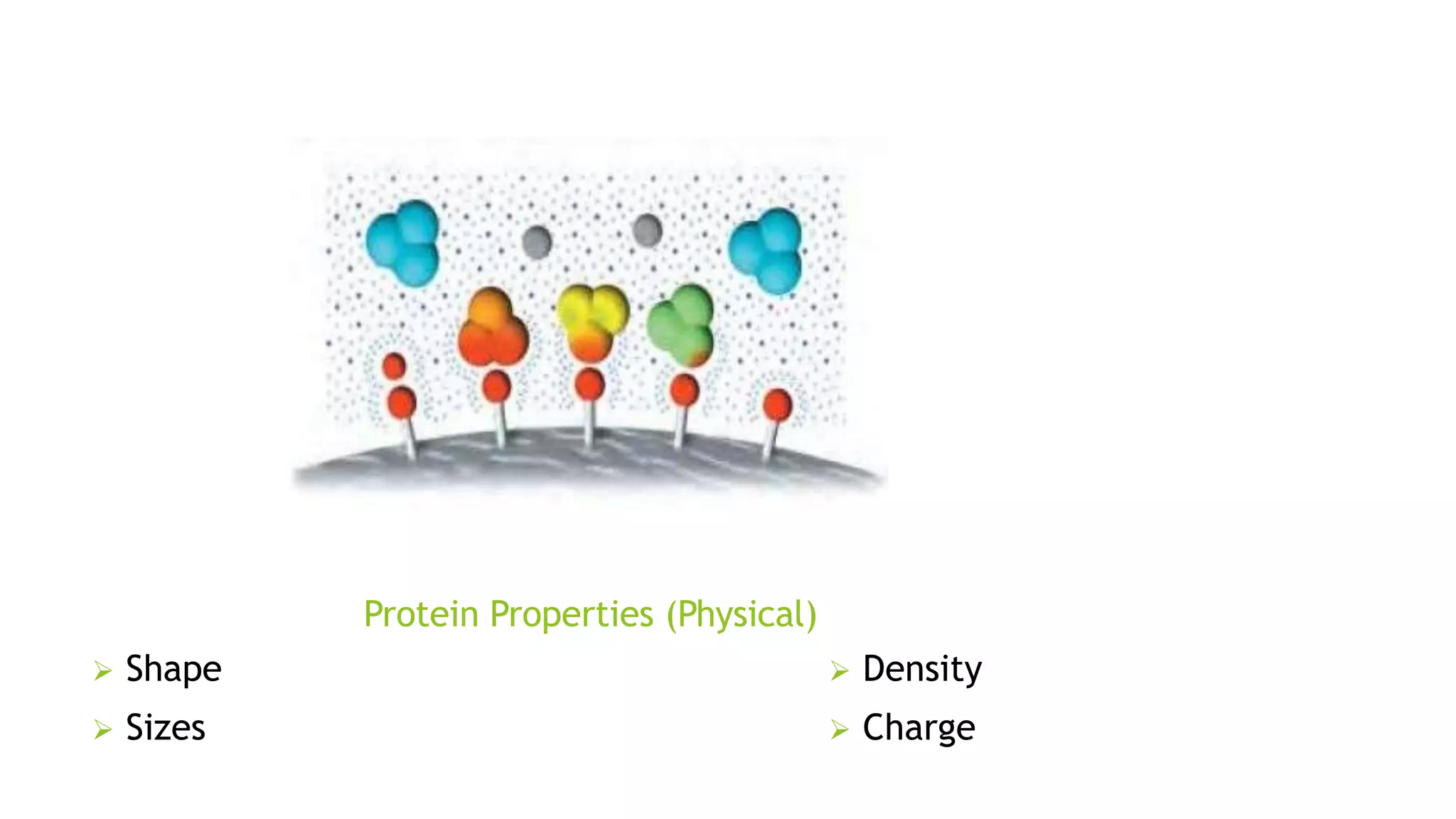
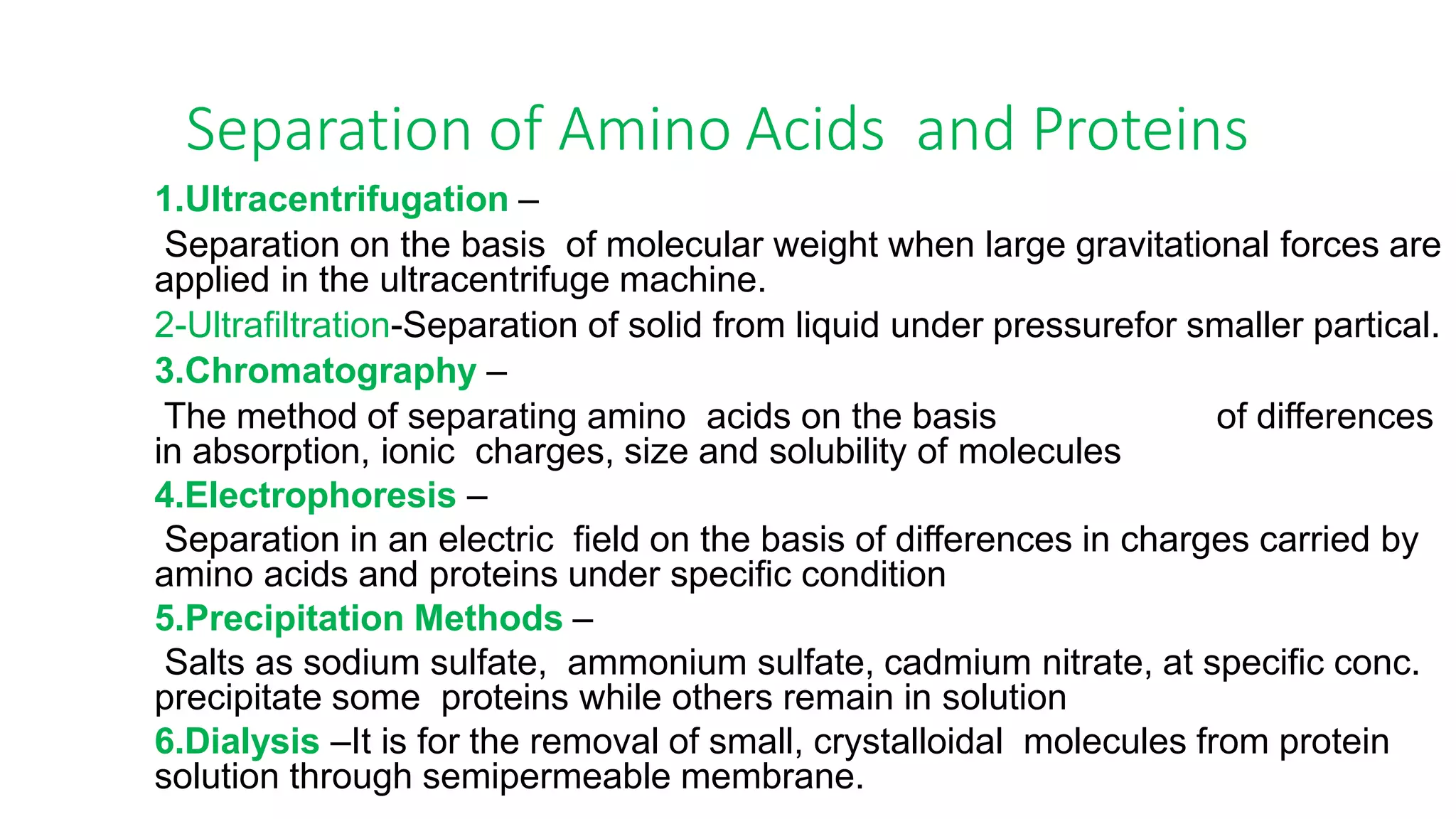
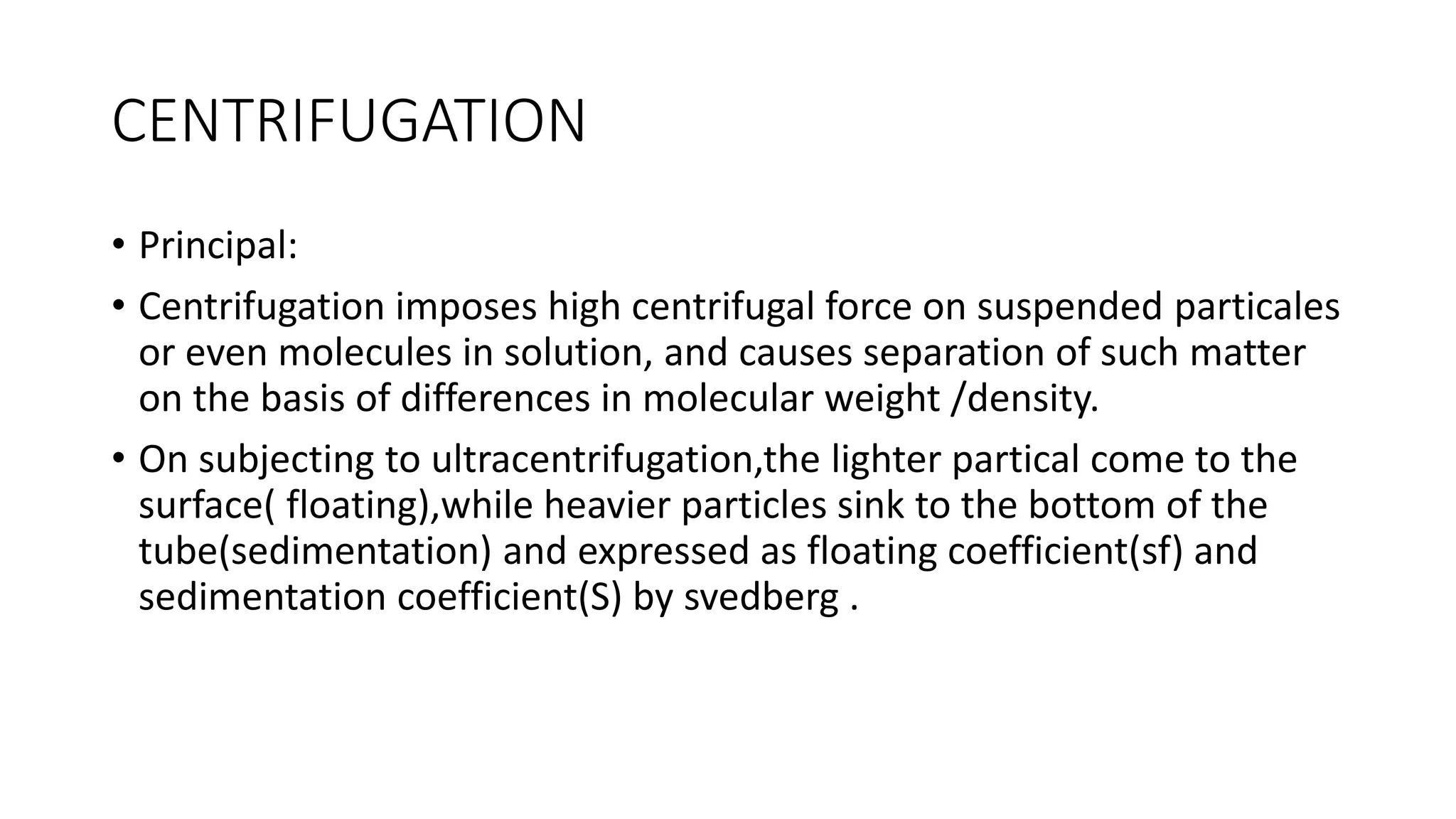
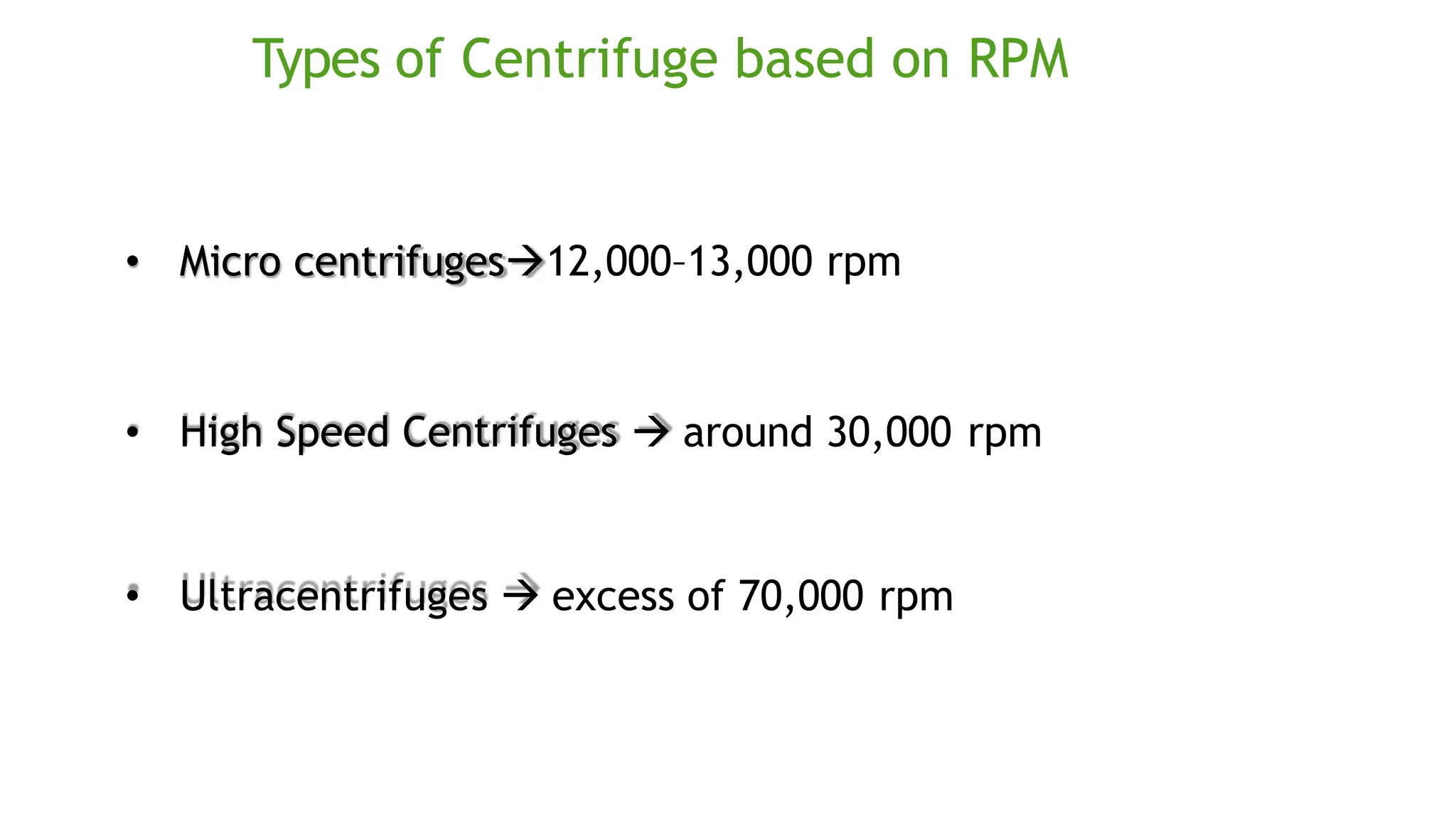
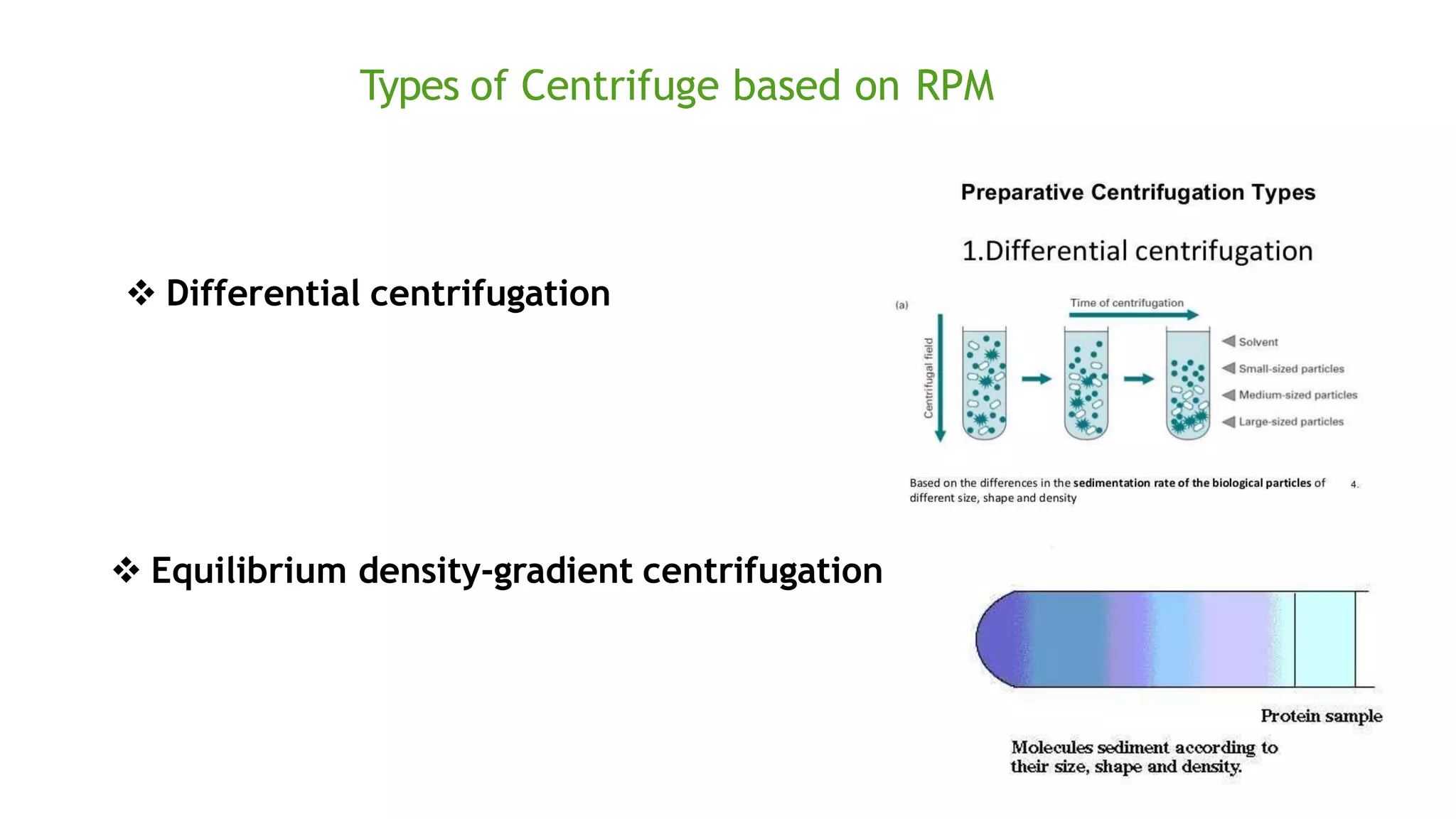
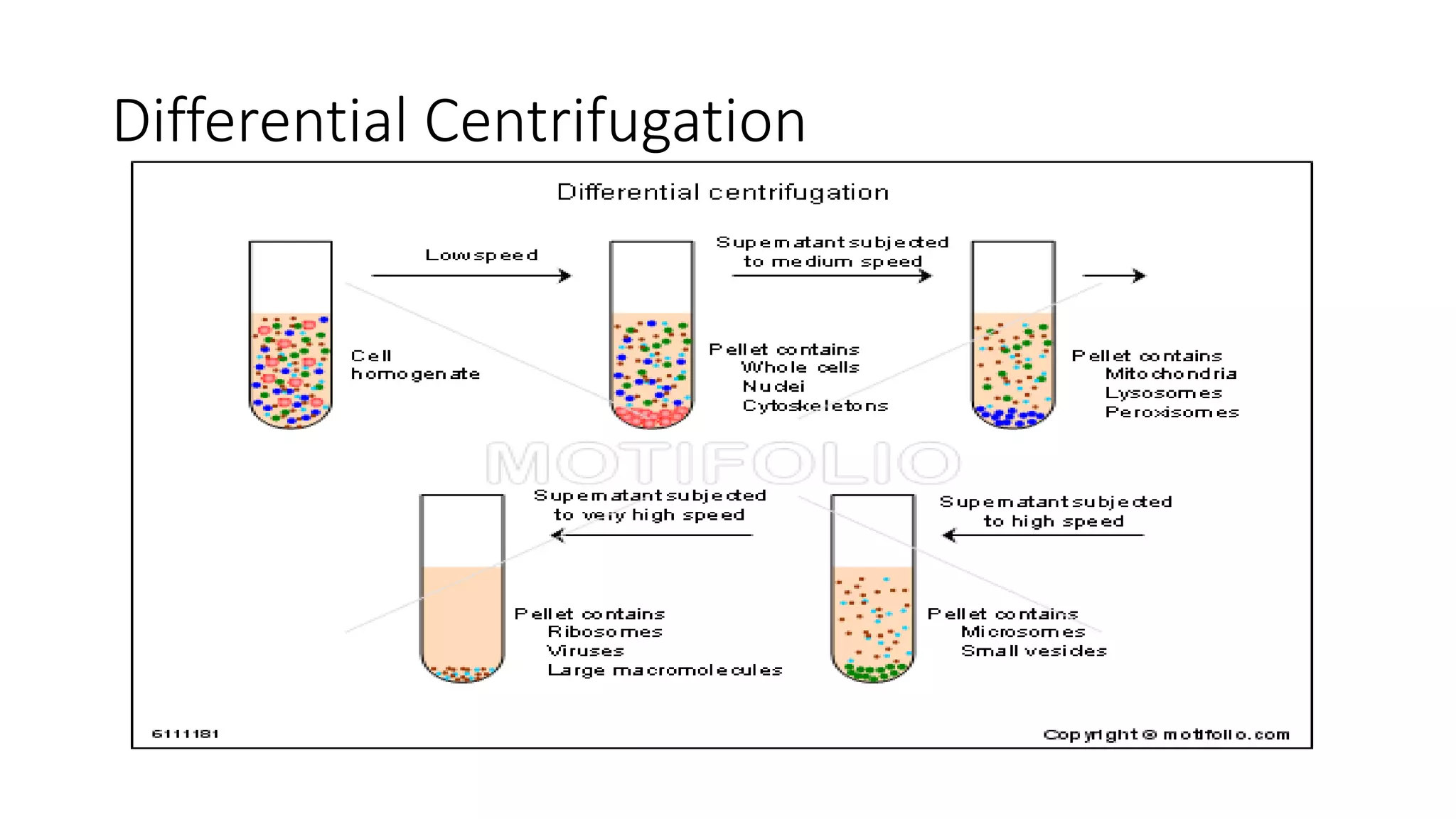
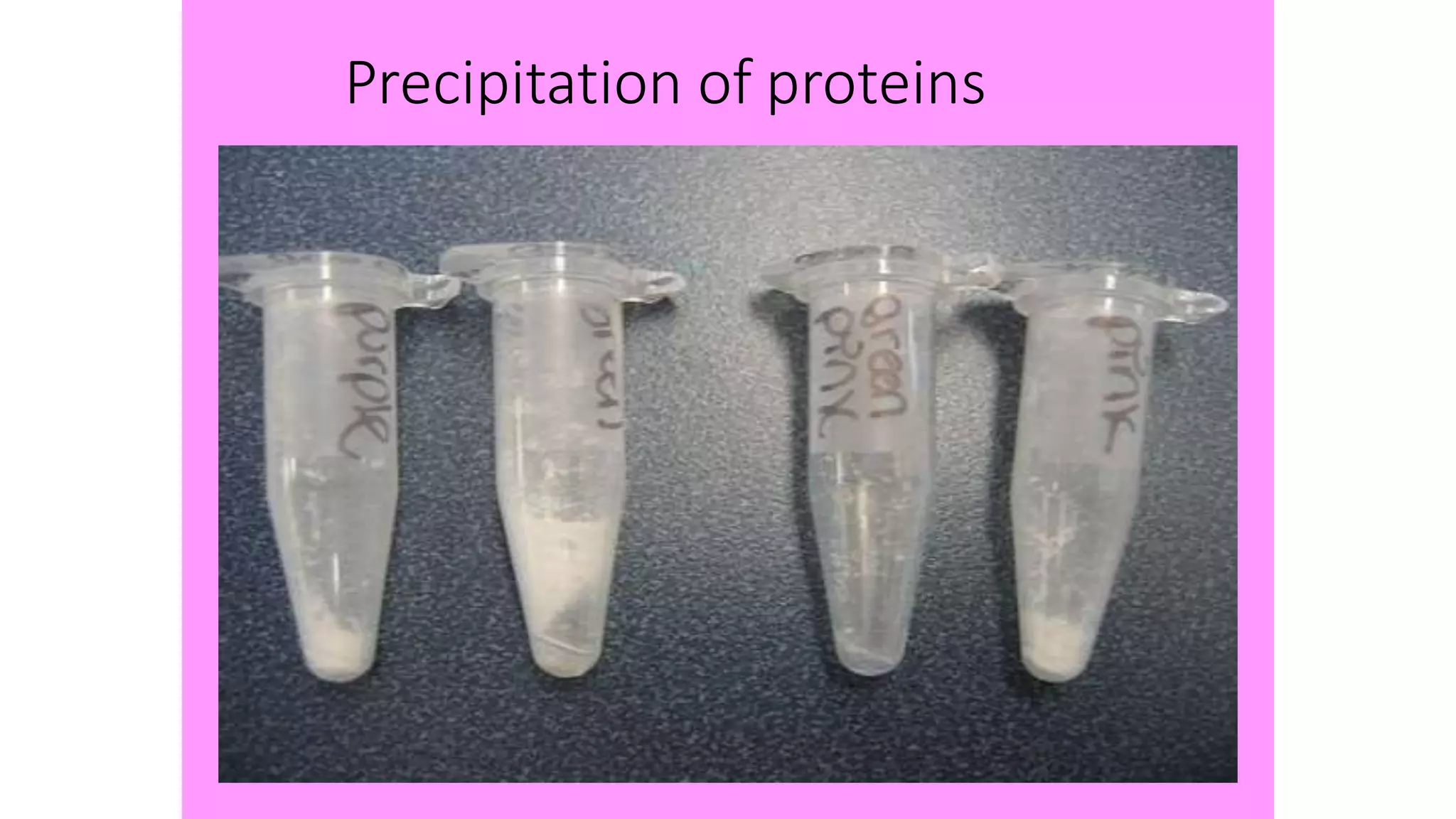
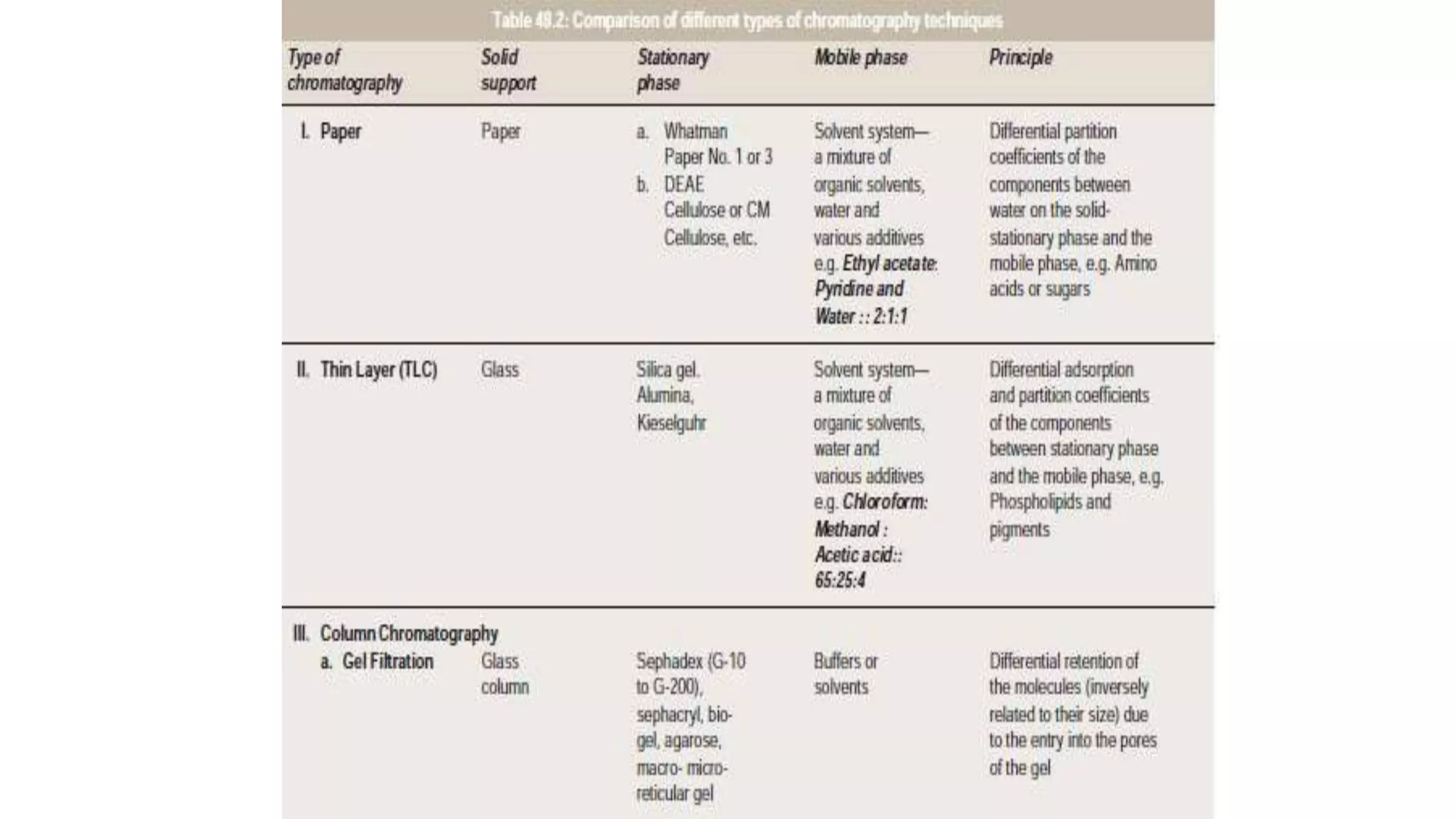
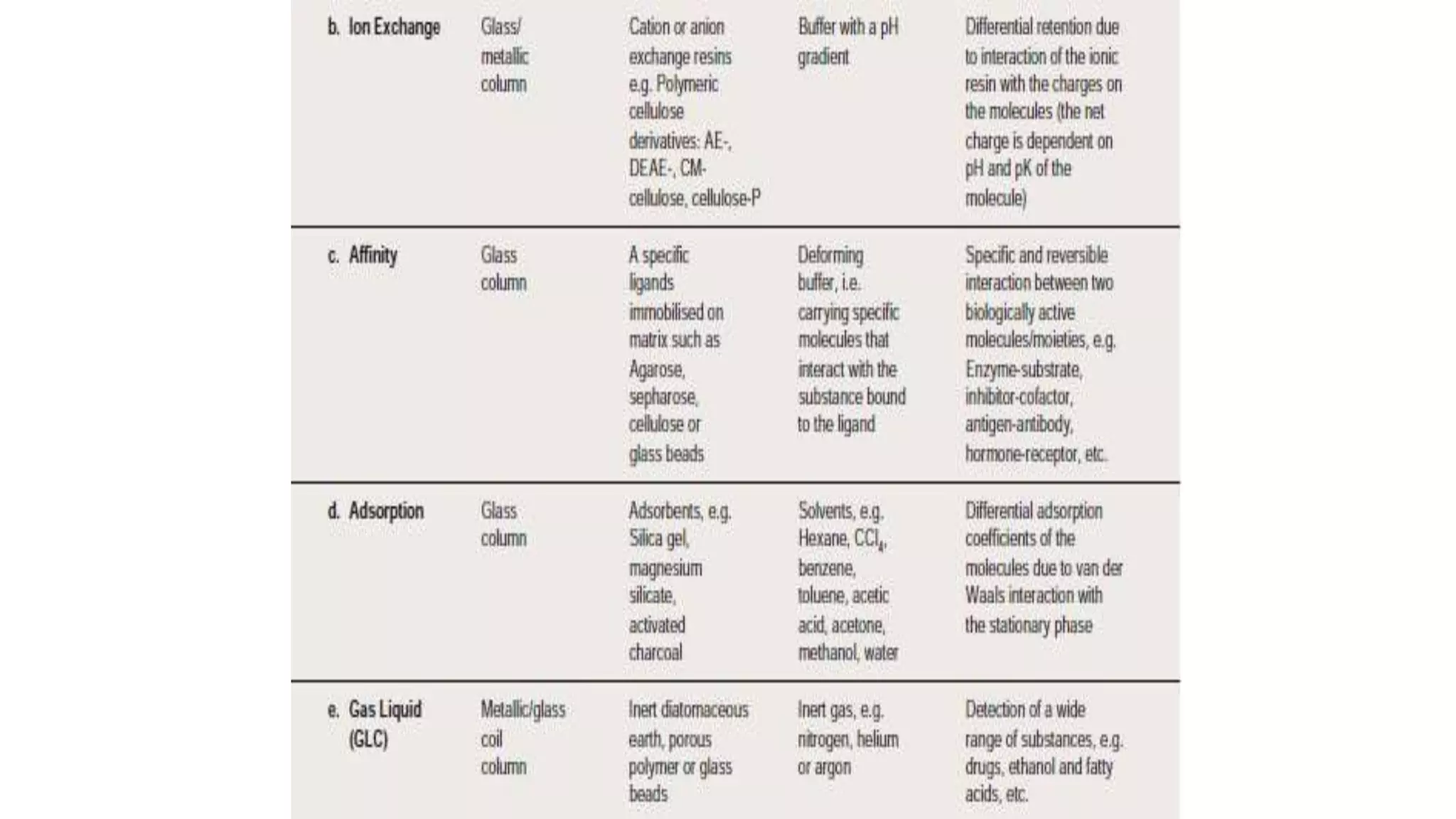
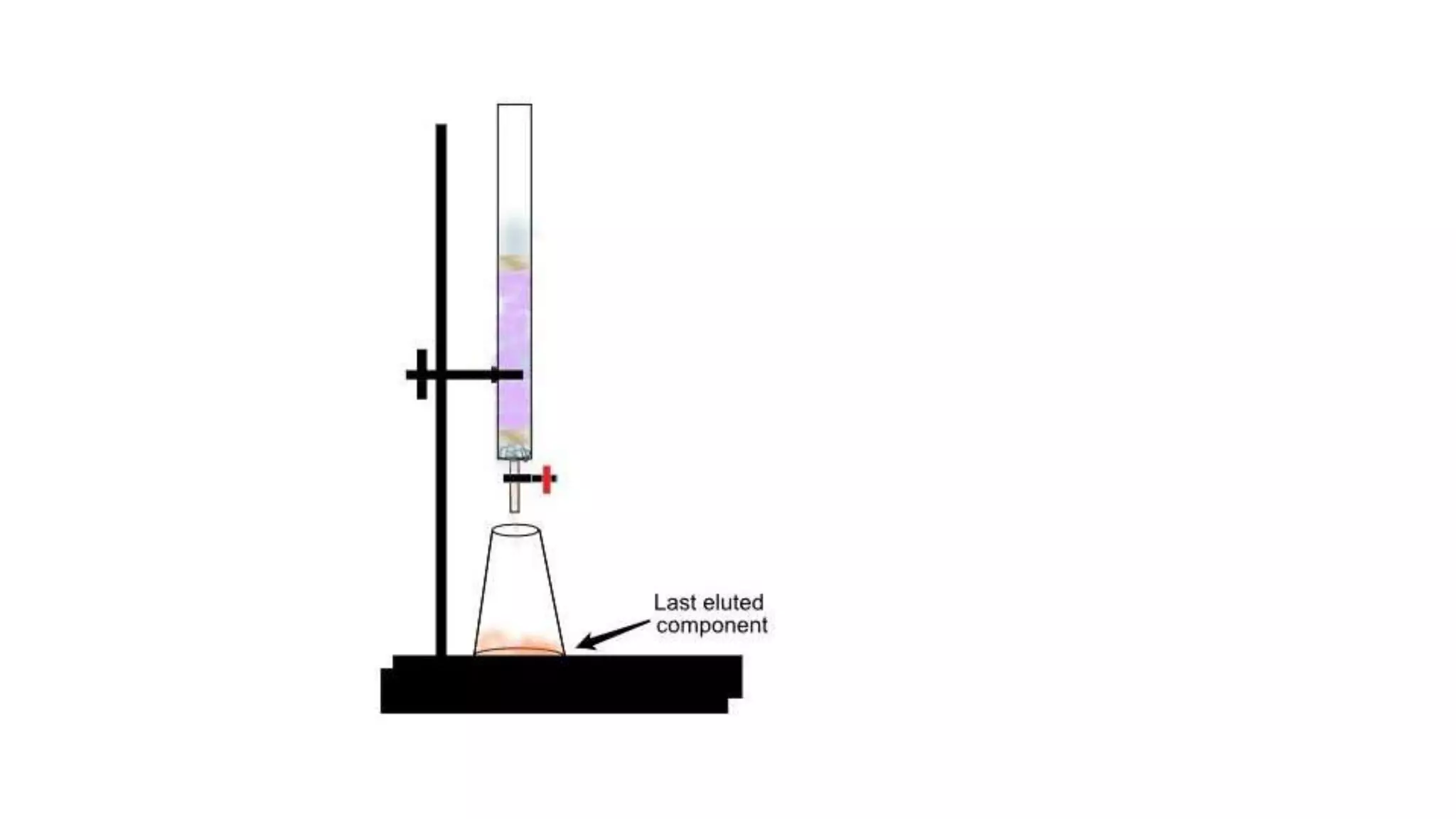
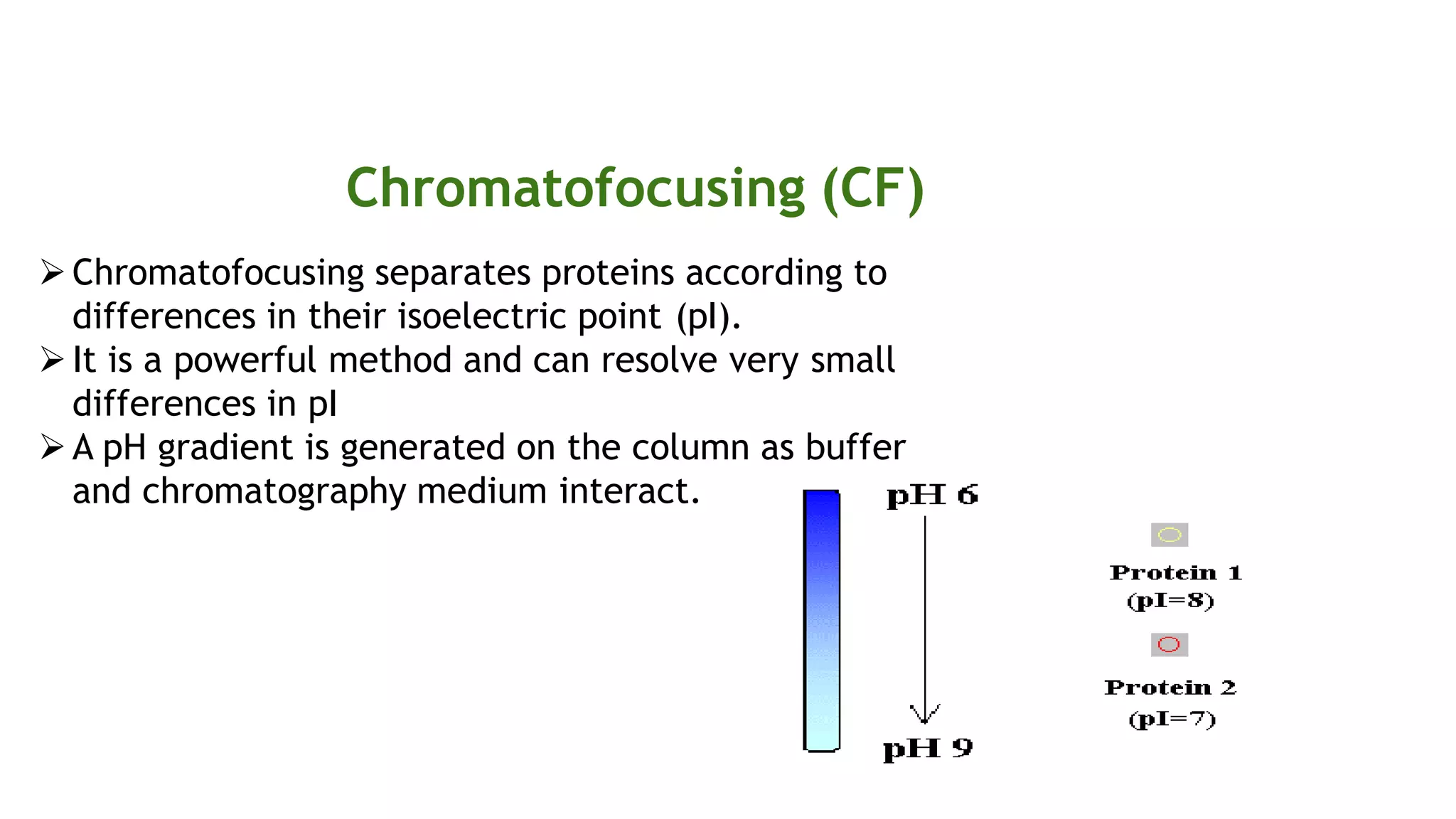
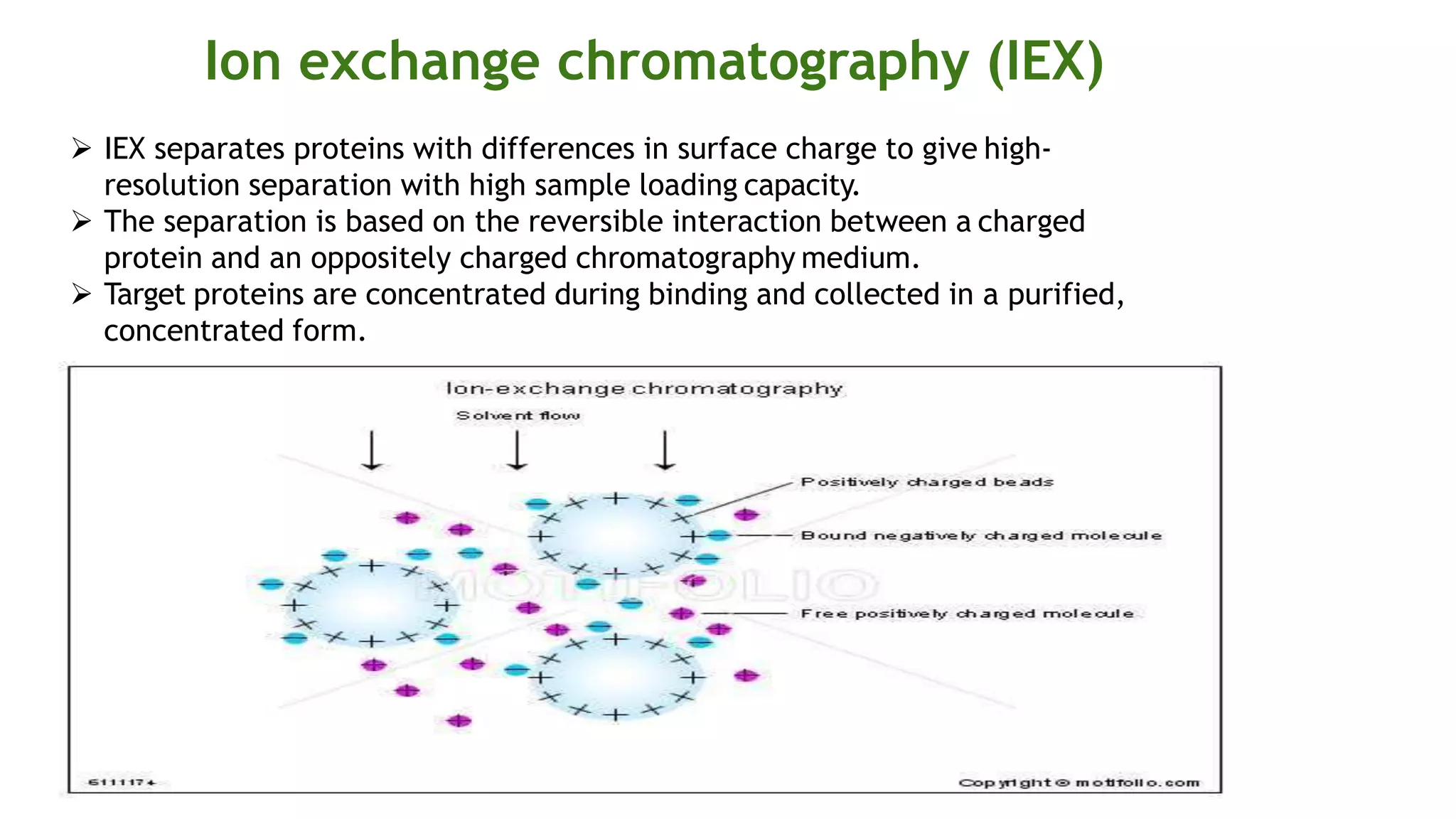
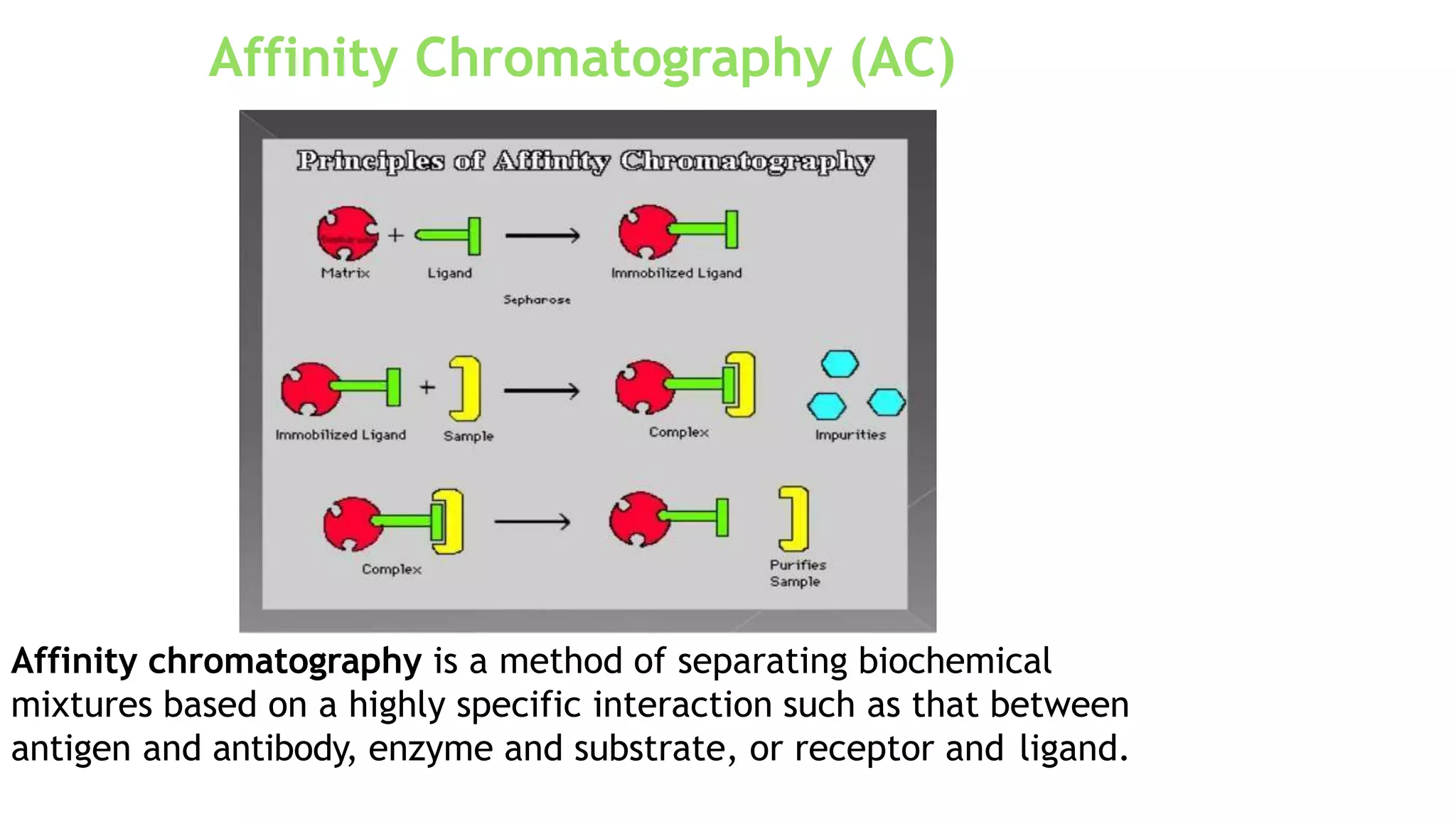
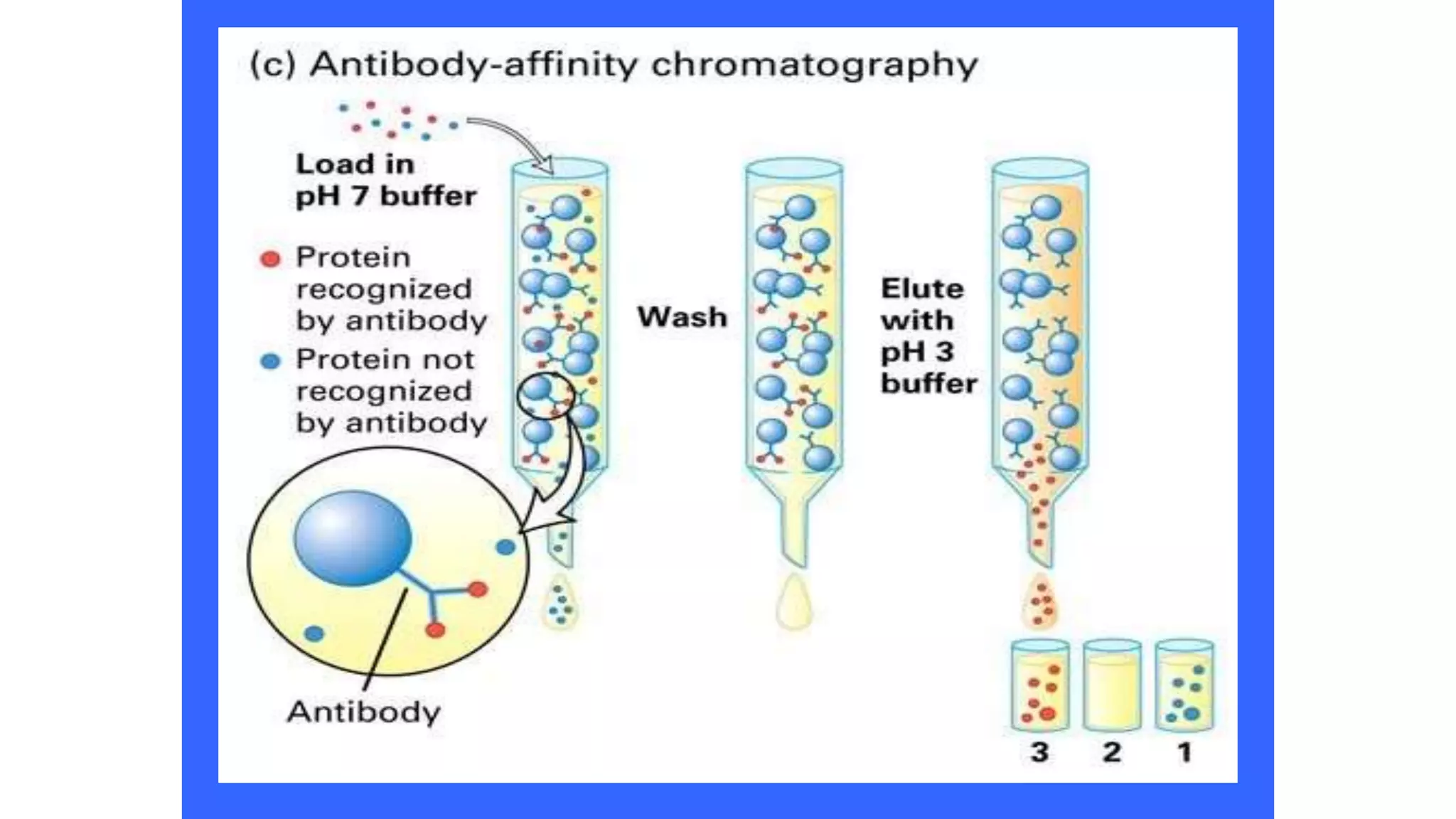
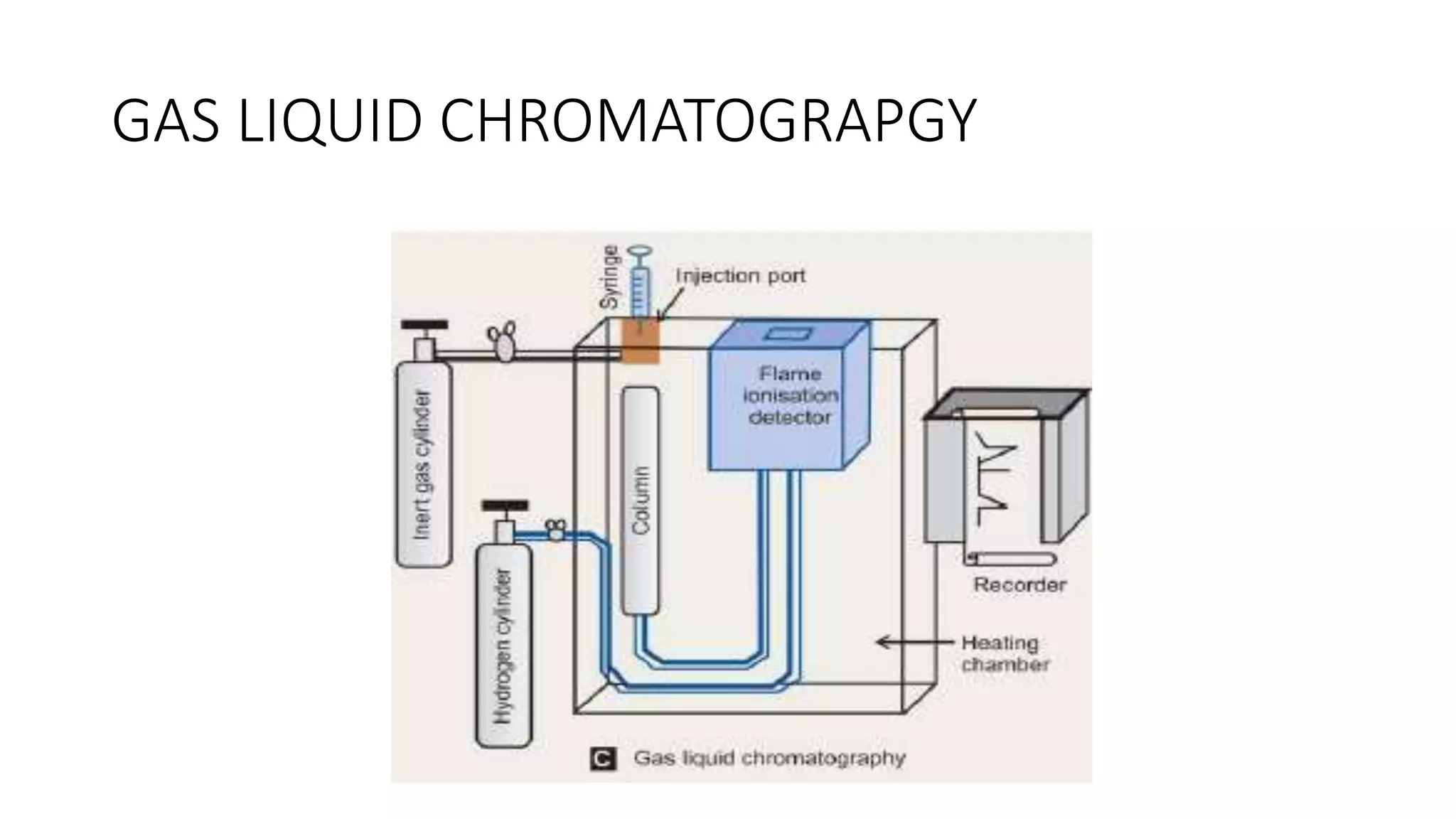
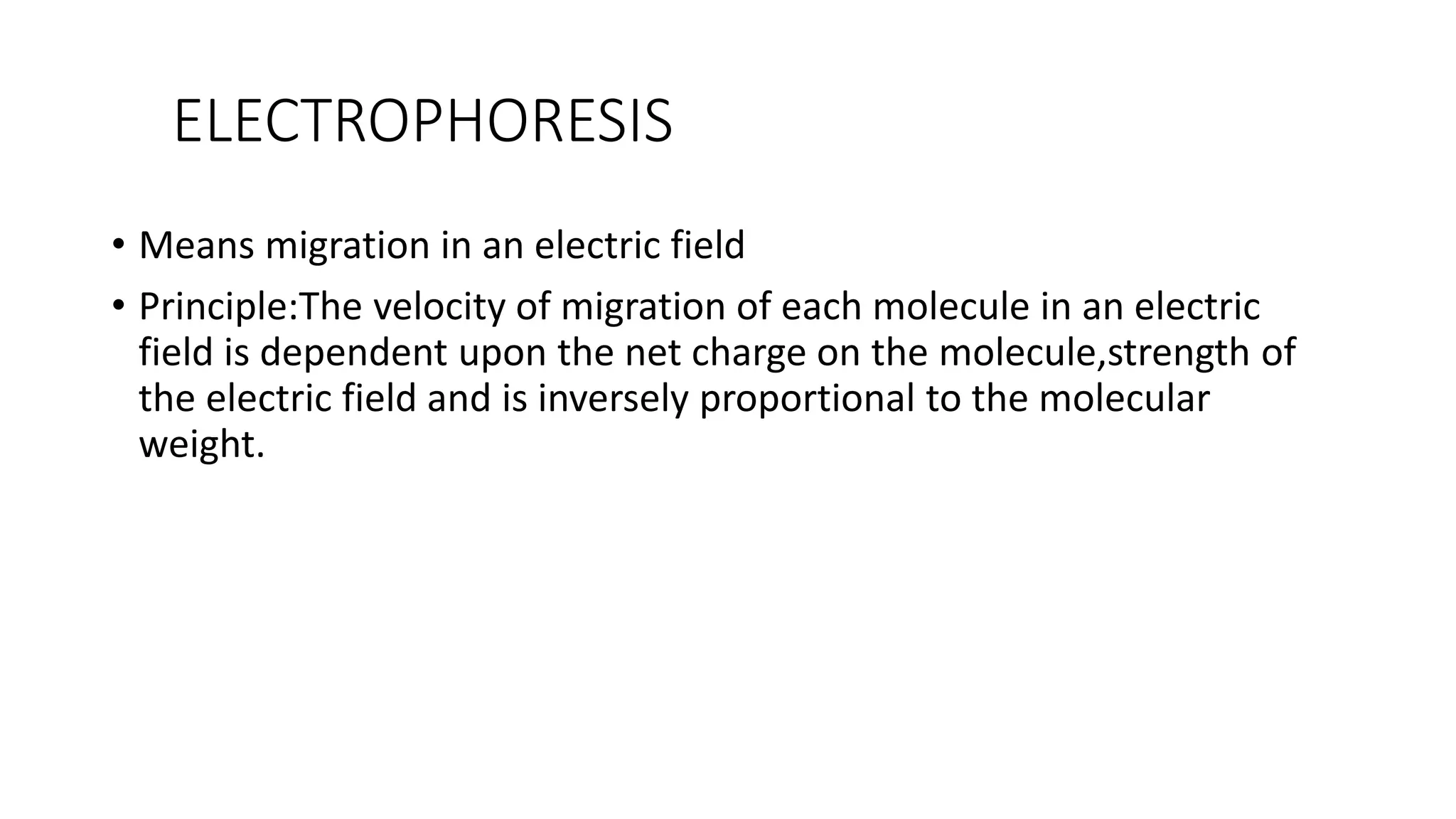
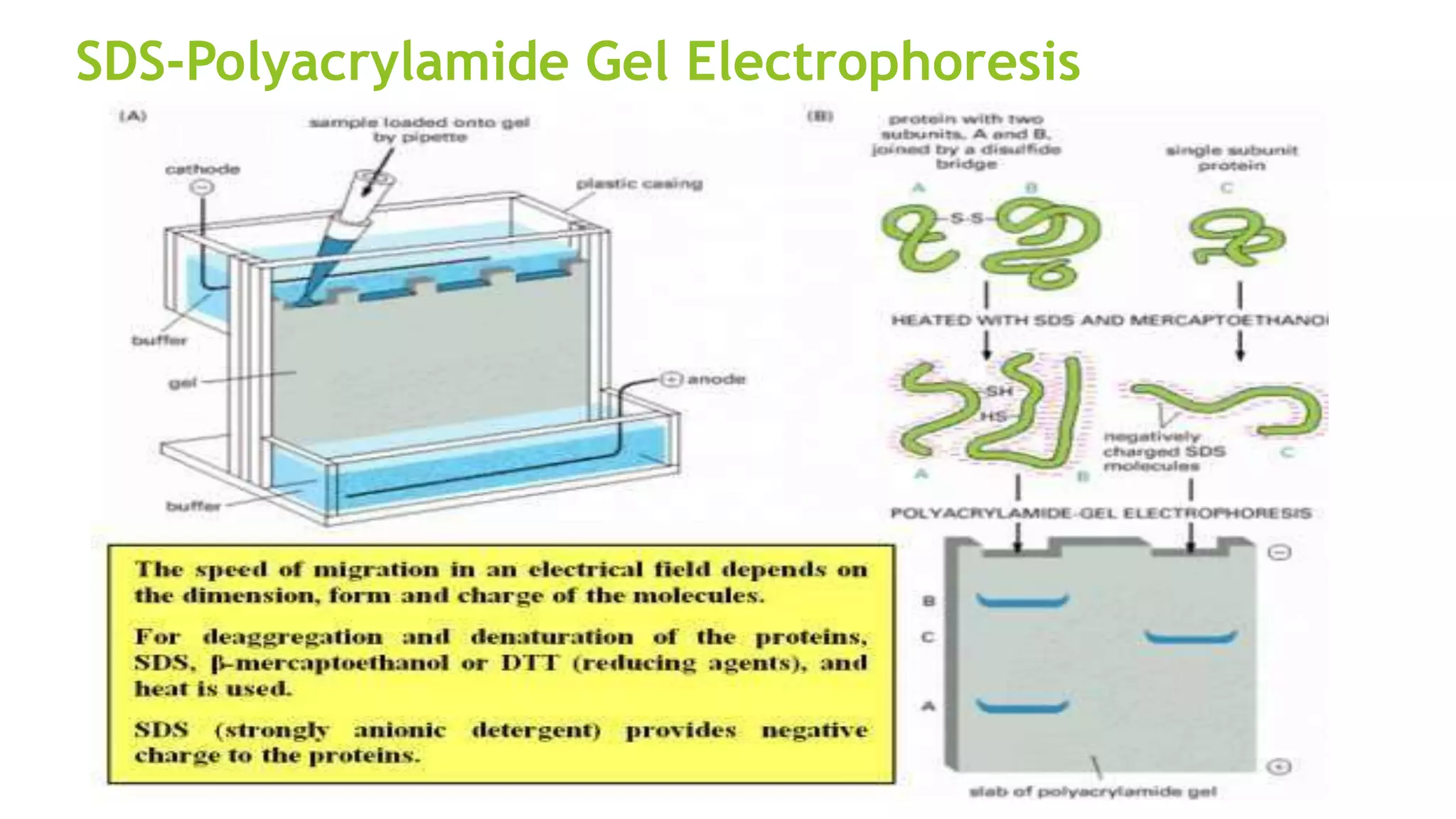
Protein purification involves a series of processes to isolate a single type of protein from a complex mixture. The starting material is usually a biological tissue or microbial culture. Various methods are used to separate the protein from non-protein parts of the mixture and finally separate the desired protein from all others. Key methods used include centrifugation, chromatography, electrophoresis, and precipitation. The goal is to purify the protein to homogeneity for characterization of its structure, function and interactions.