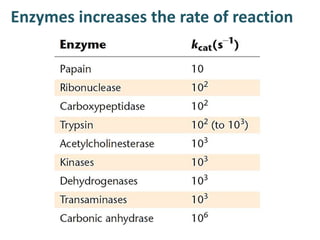
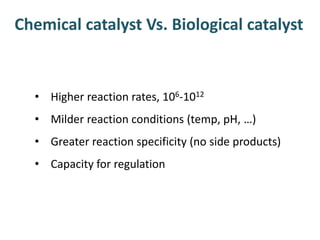
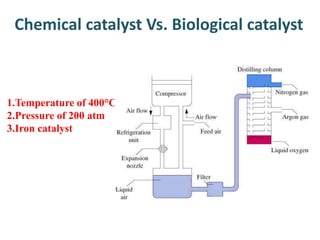
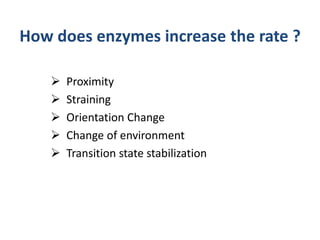
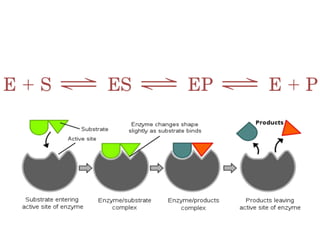
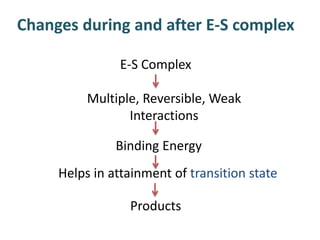
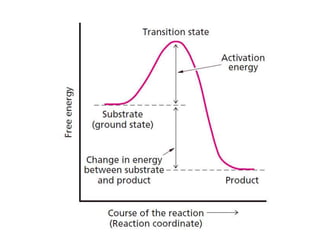
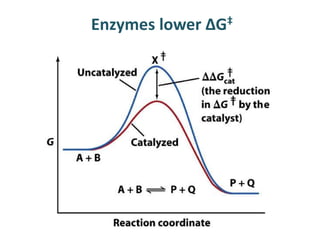
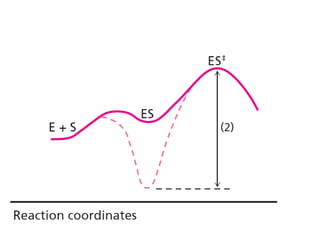
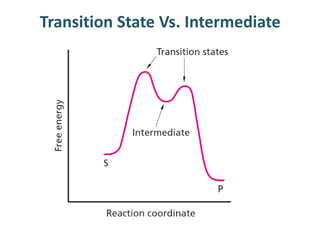
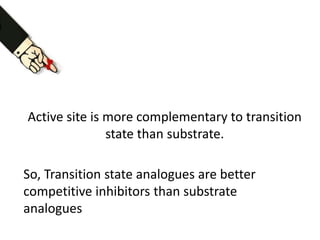
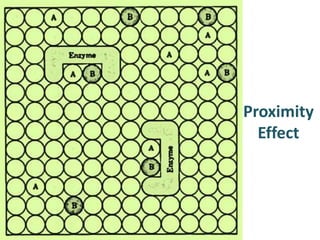
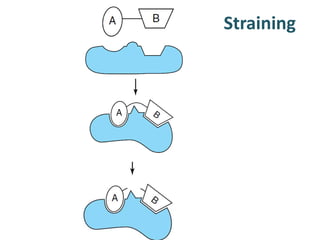
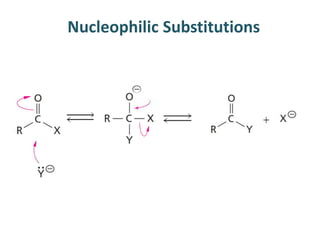
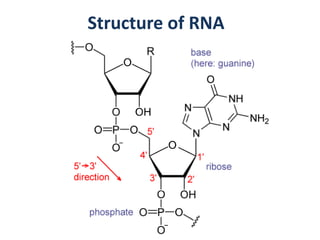
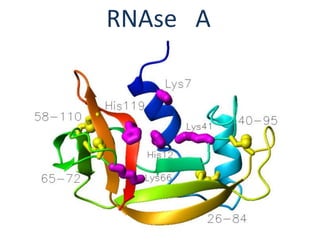
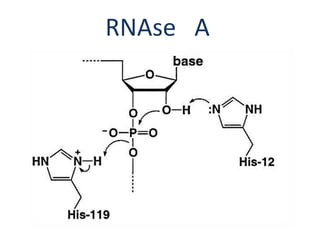
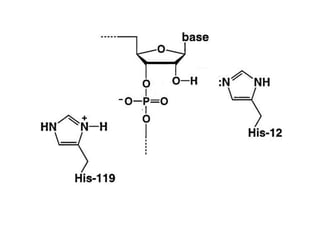
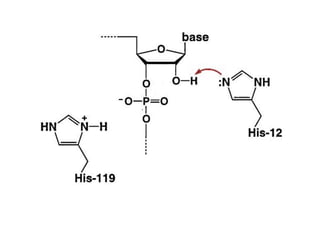
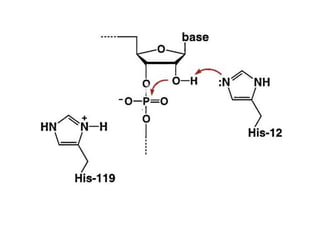
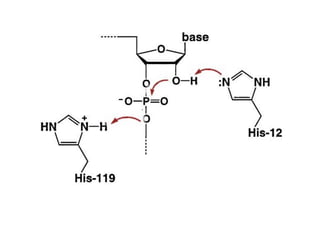
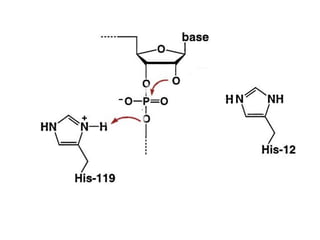
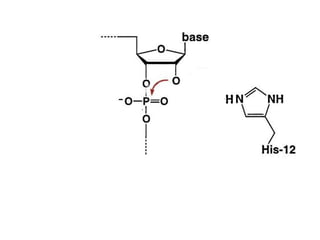
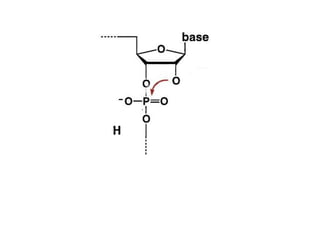
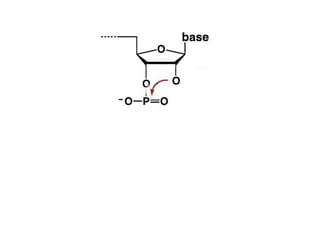
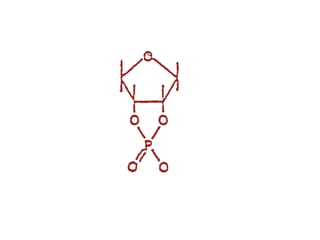
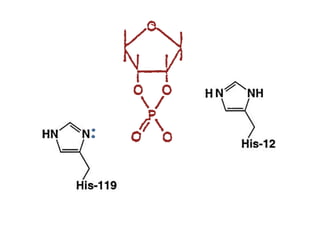
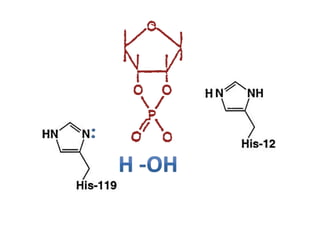
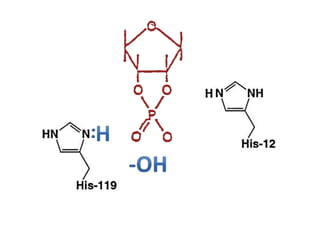
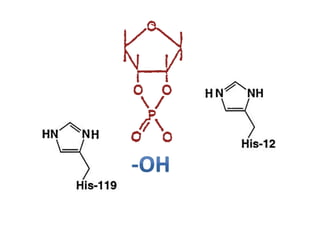
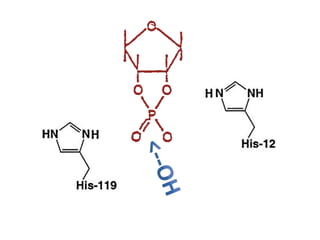
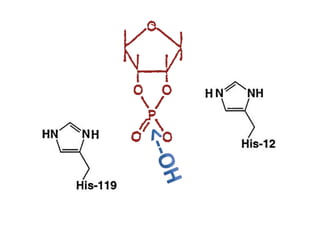
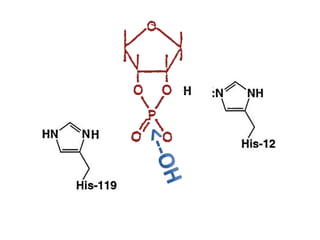
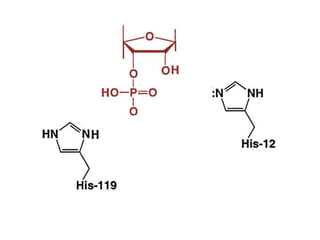
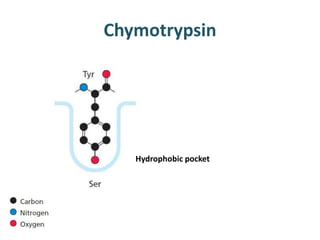
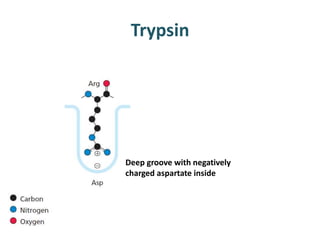
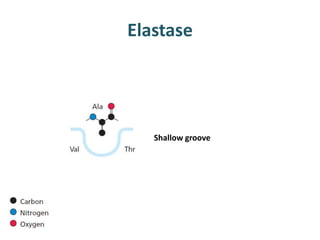
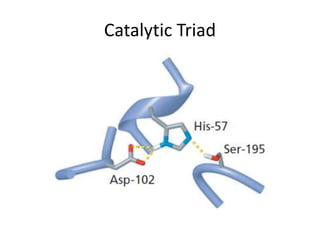
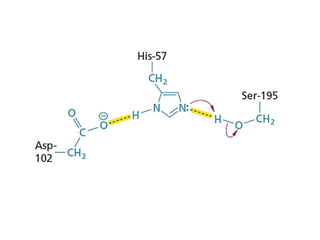
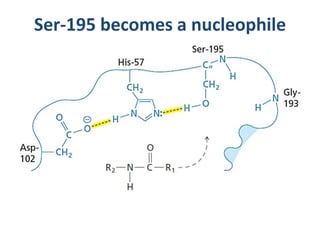
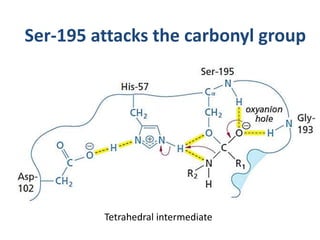
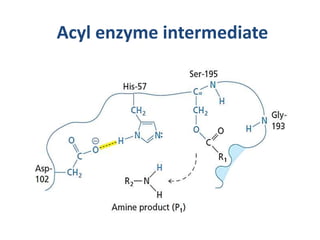
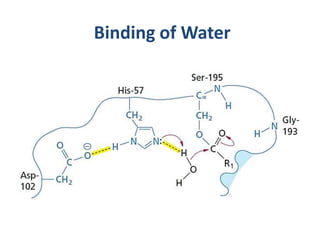
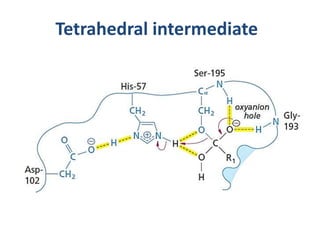
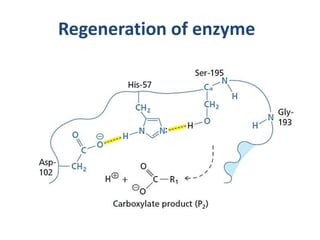
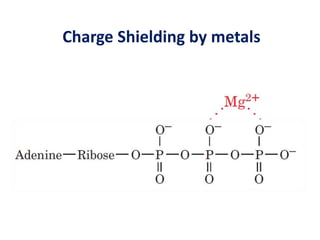
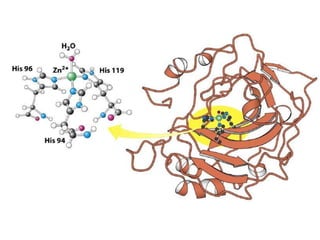
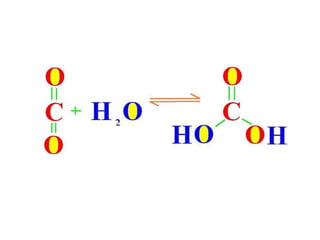
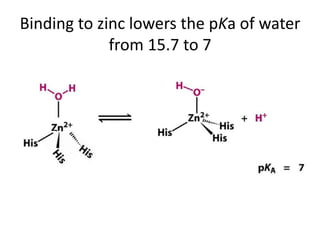
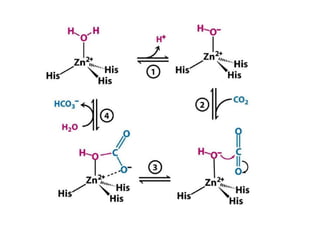
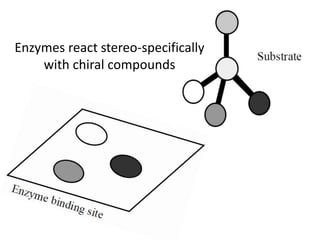
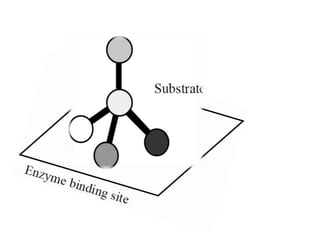
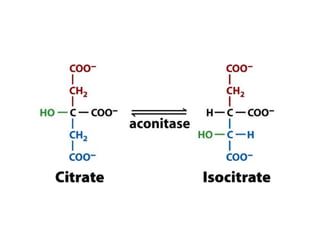
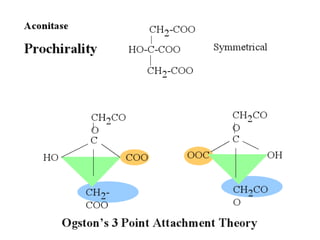
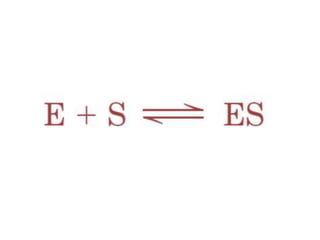The document discusses the mechanisms of enzymatic catalysis, highlighting how enzymes enhance reaction rates, specificity, and regulate conditions. It elaborates on different types of catalysis, including acid-base, covalent, and metal ion catalysis, as well as the significance of active sites and transition states in enzyme action. Examples of various proteases and their catalytic mechanisms are also provided, emphasizing their evolutionary relationships.